Question: Please solve these problems I need the correct answer ASAP Question 9 O out of o 35 points The liquid phase irreversible reaction 2A -
Please solve these problems I need the correct answer ASAP
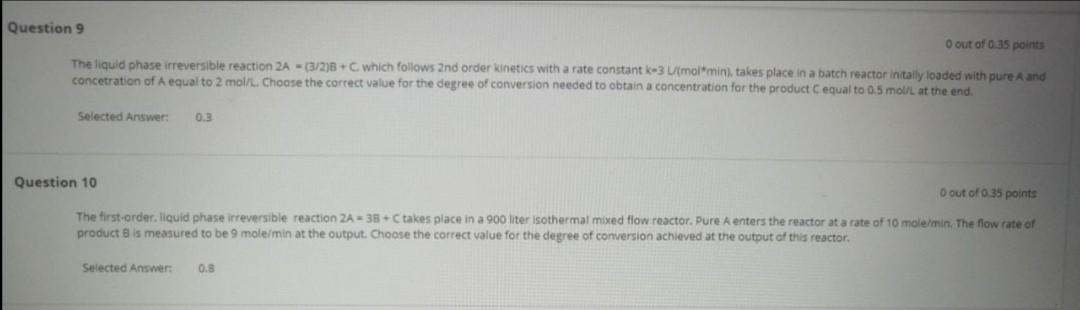
Question 9 O out of o 35 points The liquid phase irreversible reaction 2A - (3/2)6+ which follows 2nd order kinetics with a rate constant-3 (molmin), takes place in a batch reactor intally loaded with pure A and concetration of A equal to 2 mol/l. Choose the correct value for the degree of conversion needed to obtain a concentration for the product Cequal to 0.5 mol/L at the end. Selected Answer: 0.3 Question 10 O out of 35 points The first-order, liquid phase irreversible reaction ZA = 38C takes place in a 900 liter isothermal mixed flow reactor. Pure A enters the reactor at a rate of 10 mole/min. The flow rate of product is measured to be 9 mole/min at the output. Choose the correct value for the degree of conversion achieved at the output of this reactor. Selected Answers 0.8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


