Question: Please solve these question in details and fully explaination.Because it is the prepare for lab, so in case for those data you needed, please just
Please solve these question in details and fully explaination.Because it is the prepare for lab, so in case for those data you needed, please just assume it as x, y, z and explain step by step, formula, etc. for me to do it. Thank you so much in advance.
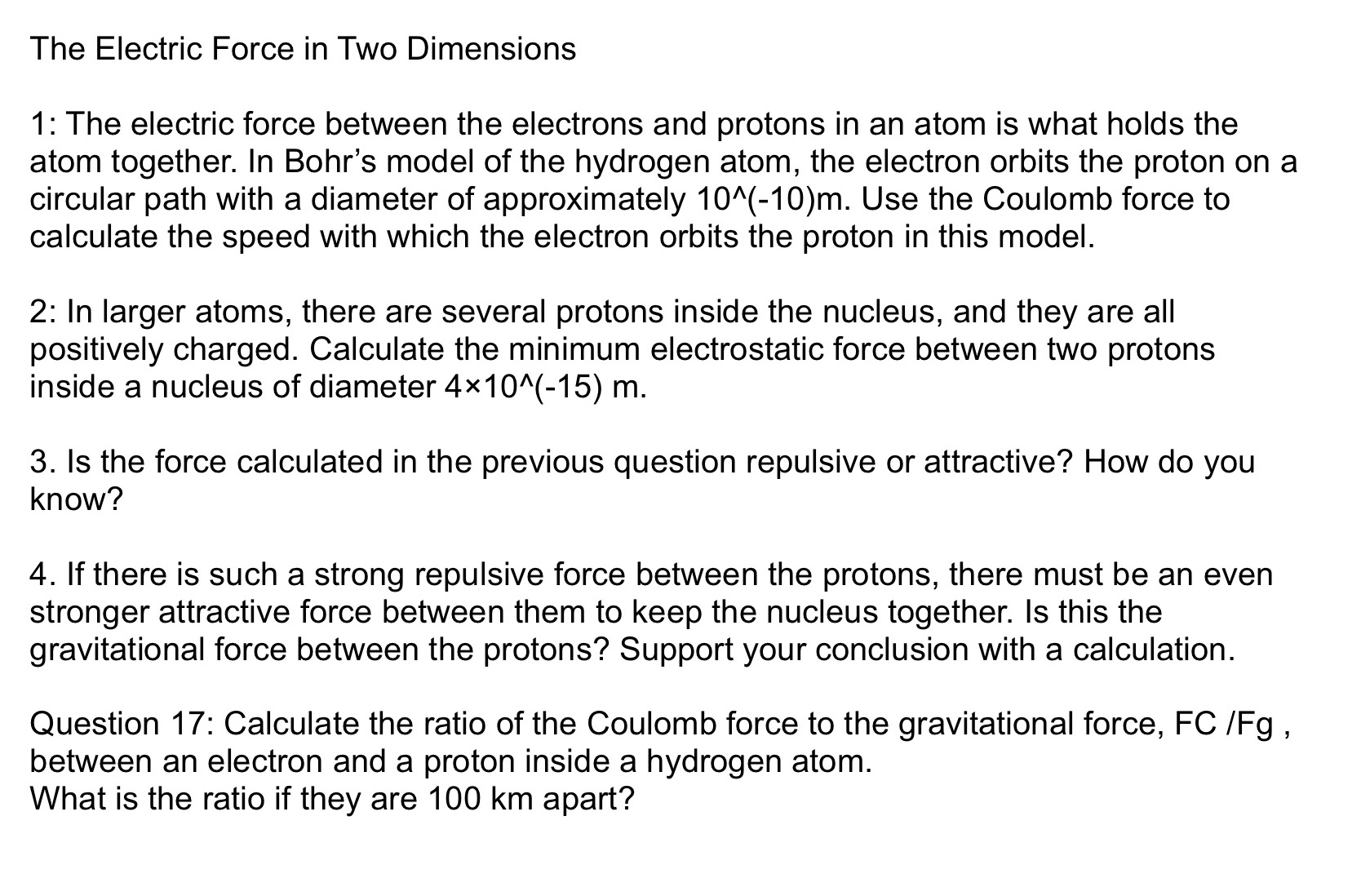
The Electric Force in Two Dimensions 1: The electric force between the electrons and protons in an atom is what holds the atom together. In Bohr's model of the hydrogen atom, the electron orbits the proton on a circular path with a diameter of approximately 10"(-10)m. Use the Coulomb force to calculate the speed with which the electron orbits the proton in this model. 2: In larger atoms, there are several protons inside the nucleus, and they are all positively charged. Calculate the minimum electrostatic force between two protons inside a nucleus of diameter 4X10"(-15) m. 3. Is the force calculated in the previous question repulsive or attractive? How do you know? 4. If there is such a strong repulsive force between the protons, there must be an even stronger attractive force between them to keep the nucleus together. Is this the gravitational force between the protons? Support your conclusion with a calculation. Question 17: Calculate the ratio of the Coulomb force to the gravitational force, FC /Fg , between an electron and a proton inside a hydrogen atom. What is the ratio if they are 100 km apart
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


