Question: please solve this problem and explain how did you do it. do not copy other tutor's solution please 2-68 OEQ (Old Exam Question) Taken from

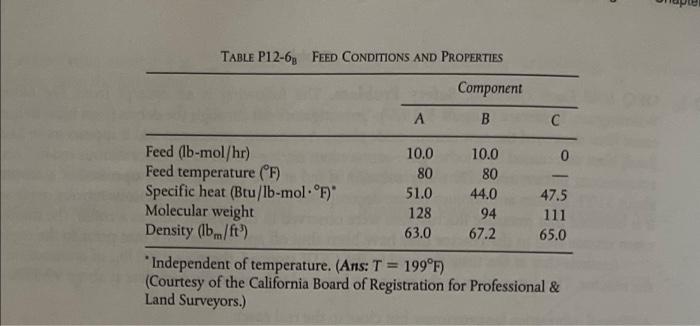
2-68 OEQ (Old Exam Question) Taken from California Professional Engineer's Exam. The endother- mic liquid-phase elementary reaction A+B-20 proceeds, substantially, to completion in a single steam-jacketed, continuous-stirred reac- tor (Table P12-6p). From the following data, calculate the steady-state reactor tempera- ture: Reactor volume: 125 gal Steam jacket area: 10 ft2 Jacket steam: 150 psig (365.9F saturation temperature) Overall heat-transfer coefficient of jacket, U: 150 Btu/h.ft2. F Agitator shaft horsepower: 25 hp Heat of reaction, AHR = +20000 Btu/1b-mol of A (independent of temperature) , ( Table P12-6, FEED CONDITIONS AND PROPERTIES Component B Feed (b-mol/hr) 10.0 10.0 0 Feed temperature (F) 80 80 Specific heat (Btu/Ib-mol.F) 51.0 44.0 47.5 Molecular weight 128 94 111 Density (bm/ft) 63.0 67.2 65.0 Independent of temperature. (Ans: T = 199F) (Courtesy of the California Board of Registration for Professional & Land Surveyors.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


