Question: please solve this problem. and please answers on online. I hate hand-writing answers. Consider the following reaction: Mg(s)+HCl(aq)H2(g)+MgCl2(aq) a) Of 12.67g of magnesium react with
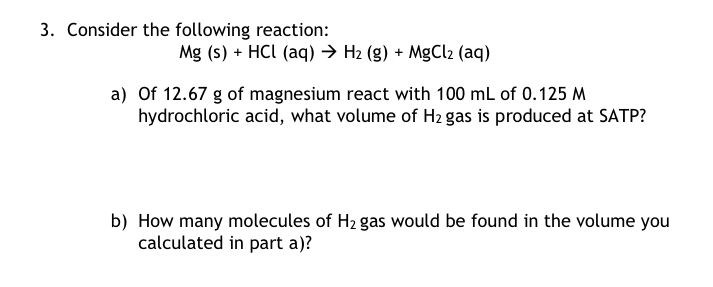
please solve this problem.
and please answers on online.
I hate hand-writing answers.
Consider the following reaction: Mg(s)+HCl(aq)H2(g)+MgCl2(aq) a) Of 12.67g of magnesium react with 100mL of 0.125M hydrochloric acid, what volume of H2 gas is produced at SATP? b) How many molecules of H2 gas would be found in the volume you calculated in part a)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


