Question: Please solve this question using EXCEL , as this problem is for an excel introductory class for engineering students. Please provide screenshots of your excel
Please solve this question using EXCEL, as this problem is for an excel introductory class for engineering students.
Please provide screenshots of your excel workbook, showing your step-by-step solution.
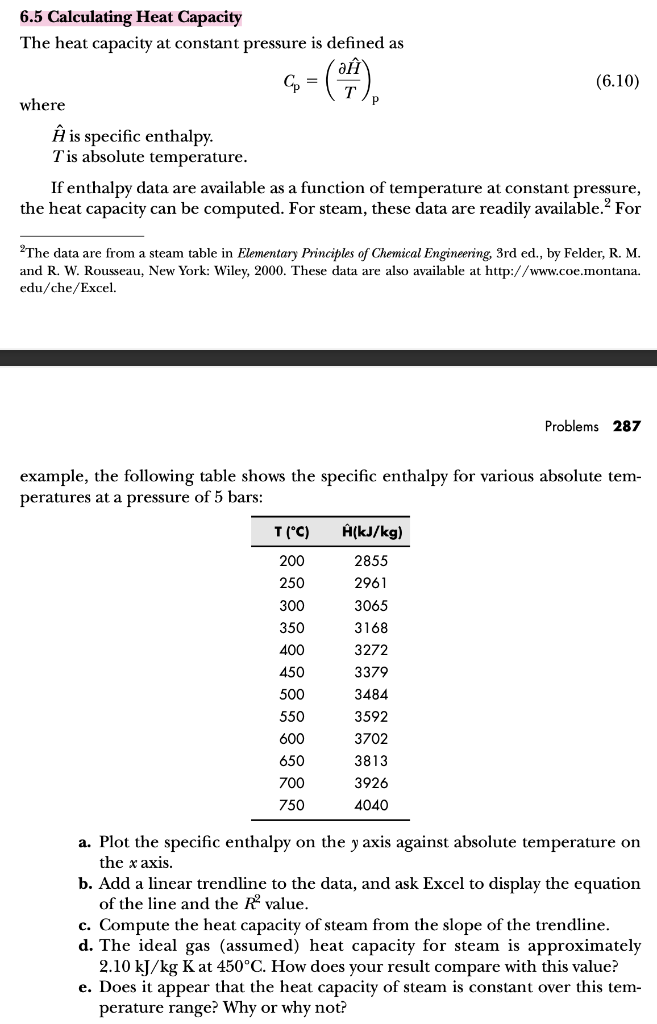
6.5 Calculating Heat Capacity The heat capacity at constant pressure is defined as Cp=(TH^)p where H^ is specific enthalpy. T is absolute temperature. If enthalpy data are available as a function of temperature at constant pressure, the heat capacity can be computed. For steam, these data are readily available. 2 For 2 The data are from a steam table in Elementary Principles of Chemical Engineering, 3rd ed., by Felder, R. M. and R. W. Rousseau, New York: Wiley, 2000. These data are also available at http://www.coe.montana. edu/che/Excel. Problems 287 example, the following table shows the specific enthalpy for various absolute temperatures at a pressure of 5 bars: a. Plot the specific enthalpy on the y axis against absolute temperature on the x axis. b. Add a linear trendline to the data, and ask Excel to display the equation of the line and the R2 value. c. Compute the heat capacity of steam from the slope of the trendline. d. The ideal gas (assumed) heat capacity for steam is approximately 2.10kJ/kgK at 450C. How does your result compare with this value? e. Does it appear that the heat capacity of steam is constant over this temperature range? Why or why not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


