Question: please solve this two question Based on the following reaction, determine the mass (in g) of Fe required to transfer 54.8kJ of heat. 2Fe(s)+3CO2(g)Fe2O3(s)+3CO(g)H=24.7kJ Question
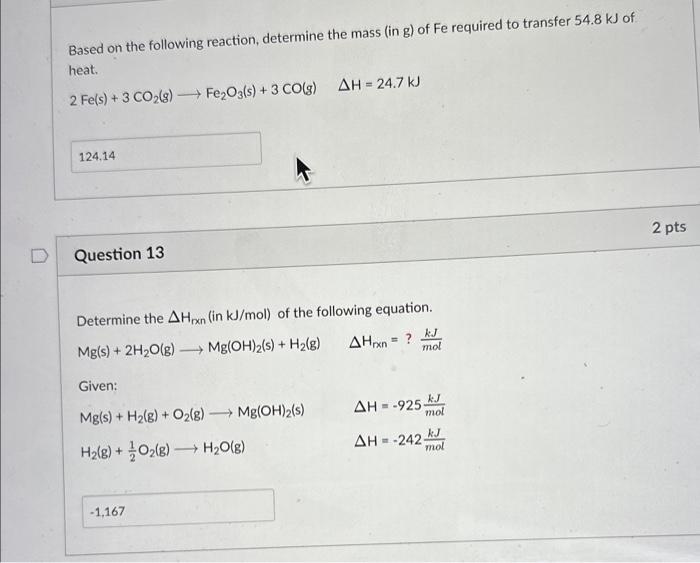
Based on the following reaction, determine the mass (in g) of Fe required to transfer 54.8kJ of heat. 2Fe(s)+3CO2(g)Fe2O3(s)+3CO(g)H=24.7kJ Question 13 Determine the Hrxn( in kJ/mol) of the following equation. Mg(s)+2H2O(g)Mg(OH)2(s)+H2(g)Hrxn=?molkJ Given: Mg(s)+H2(g)+O2(g)Mg(OH)2(s)H2(g)+21O2(g)H2O(g)H=925molkJH=242molkJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


