Question: please solve this Using power-law kinetics, the orders with respect to ethyl acetate (A) and ethanol (B) were found to be 0.92 and 0.88, respectively.
please solve this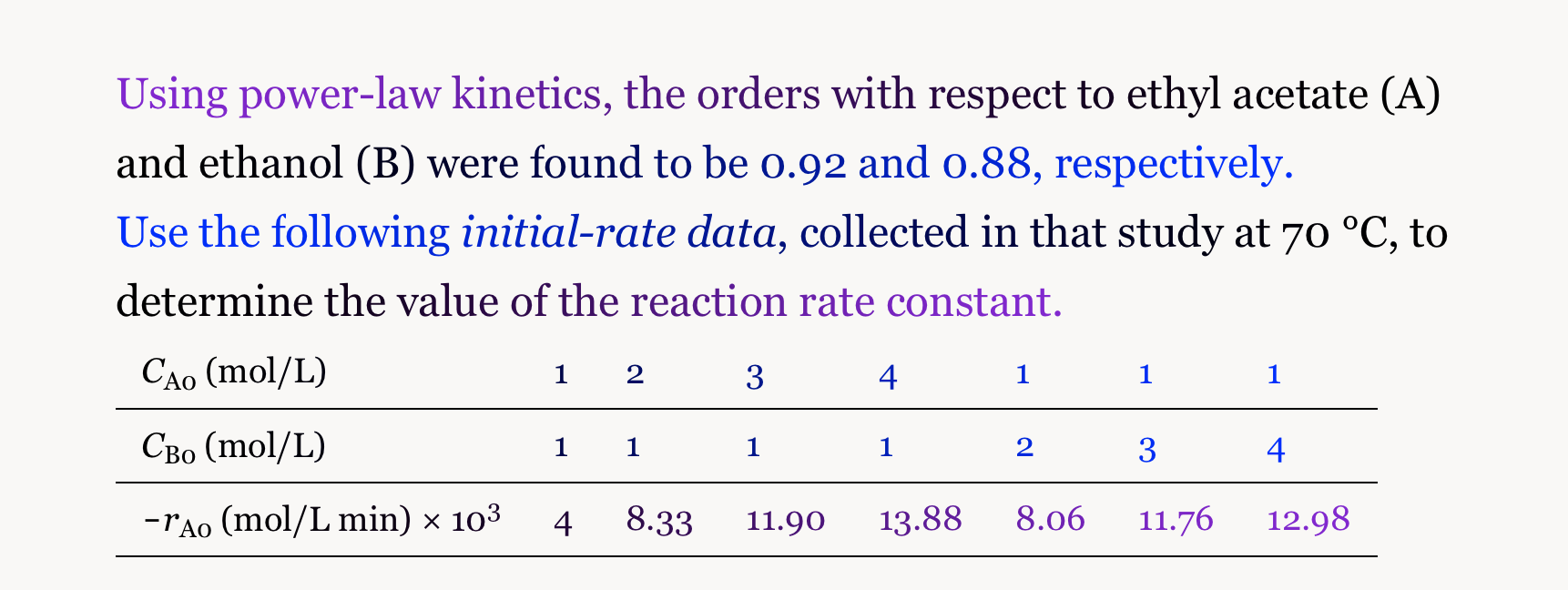
Using power-law kinetics, the orders with respect to ethyl acetate (A) and ethanol (B) were found to be 0.92 and 0.88, respectively. Use the following initial-rate data, collected in that study at 70C, to determine the value of the reaction rate constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


