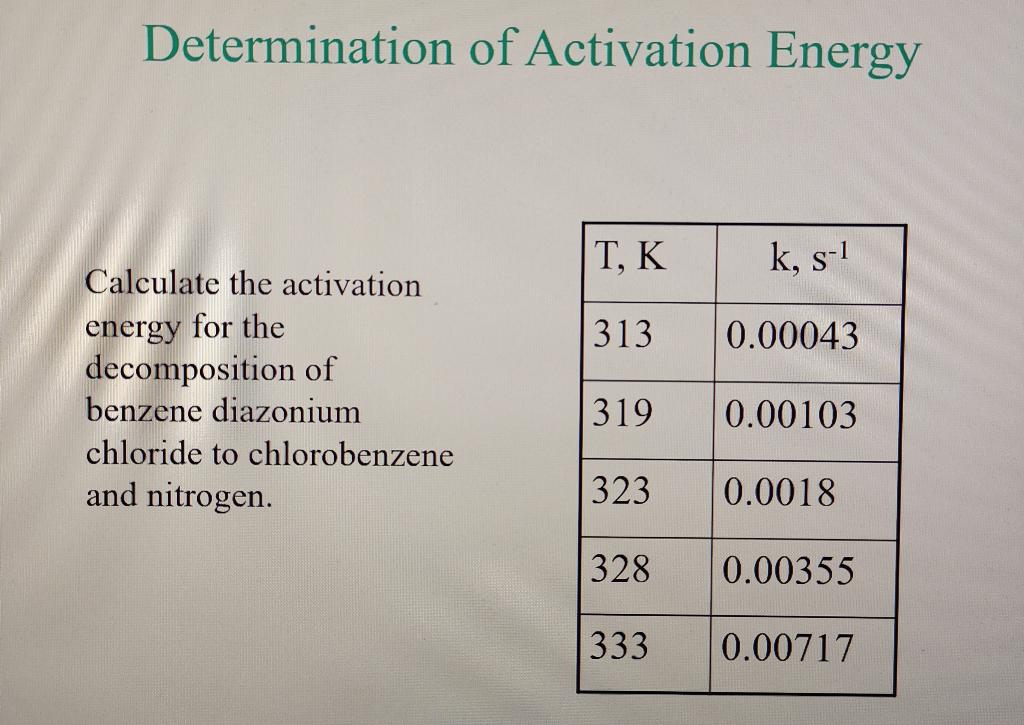
Question: please solve using LEAST SQUARE METHOD, THE ANSWER IS k=8.034x10^16exp(-121484/RT) s^-1 Determination of Activation Energy T, K k, s-1 313 0.00043 Calculate the activation energy
 please solve using LEAST SQUARE METHOD, THE ANSWER IS k=8.034x10^16exp(-121484/RT) s^-1
please solve using LEAST SQUARE METHOD, THE ANSWER IS k=8.034x10^16exp(-121484/RT) s^-1
Determination of Activation Energy T, K k, s-1 313 0.00043 Calculate the activation energy for the decomposition of benzene diazonium chloride to chlorobenzene and nitrogen. 319 0.00103 323 0.0018 328 0.00355 333 0.00717
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


