Question: please solve using tables and numerical approximation if possible. Question 3 a) A simple batch distiller is used to separate a binary mixture of A
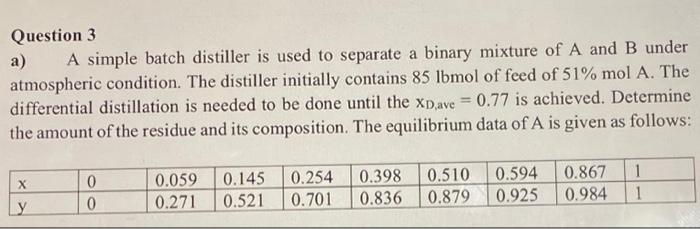
Question 3 a) A simple batch distiller is used to separate a binary mixture of A and B under atmospheric condition. The distiller initially contains 85lbmol of feed of 51%molA. The differential distillation is needed to be done until the xD,ave=0.77 is achieved. Determine the amount of the residue and its composition. The equilibrium data of A is given as follows: Question 3 a) A simple batch distiller is used to separate a binary mixture of A and B under atmospheric condition. The distiller initially contains 85lbmol of feed of 51%molA. The differential distillation is needed to be done until the xD,ave=0.77 is achieved. Determine the amount of the residue and its composition. The equilibrium data of A is given as follows
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


