Question: PLEASE SOLVE WITH DETAILS .22. Typically cooling can be accomplished by an expansion process. Consider a reversible, adiabatic expansion of an arbitrary system from total
PLEASE SOLVE WITH DETAILS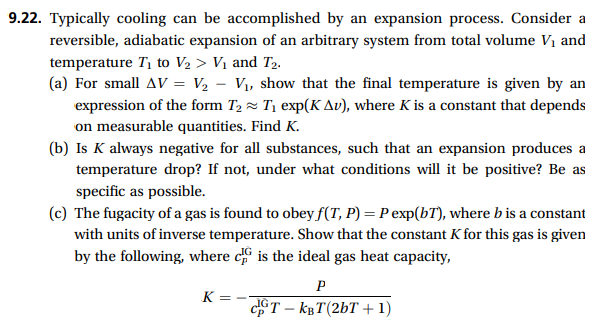
.22. Typically cooling can be accomplished by an expansion process. Consider a reversible, adiabatic expansion of an arbitrary system from total volume V1 and temperature T1 to V2>V1 and T2. (a) For small V=V2V1, show that the final temperature is given by an expression of the form T2T1exp(Kv), where K is a constant that depends on measurable quantities. Find K. (b) Is K always negative for all substances, such that an expansion produces a temperature drop? If not, under what conditions will it be positive? Be as specific as possible. (c) The fugacity of a gas is found to obey f(T,P)=Pexp(bT), where b is a constant with units of inverse temperature. Show that the constant K for this gas is given by the following, where cPIG is the ideal gas heat capacity, K=cPIGTkBT(2bT+1)P
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


