Question: Please solve within 2 hours, I will upvote! Thank you in advance!! 1. (3 points) For each of the following reactions, (1) balance the equations,
Please solve within 2 hours, I will upvote! Thank you in advance!!
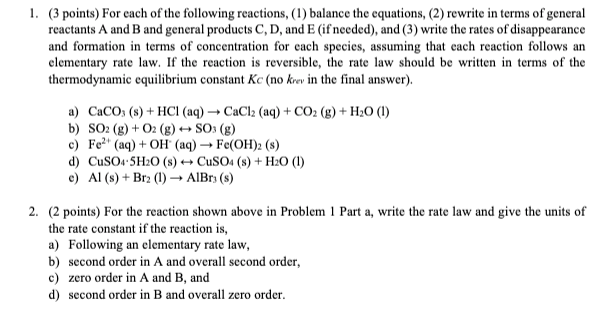
1. (3 points) For each of the following reactions, (1) balance the equations, (2) rewrite in terms of general reactants A and B and general products C, D, and E (if needed), and (3) write the rates of disappearance and formation in terms of concentration for each species, assuming that each reaction follows an elementary rate law. If the reaction is reversible, the rate law should be written in terms of the thermodynamic equilibrium constant Kc (no krev in the final answer). a) CaCO3(s) + HCl(aq) +CaCl2 (aq) + CO2(g) + H20 (1) b) SO2(g) + O2(g) - S03 (8) c) Fe2+ (aq) + OH(aq) Fe(OH)2 (8) d) CuSO4.5H20 (8) + CuSO4 (8) + H20 (1) e) A1 (8) + Bra (1) AlBr(s) 2. (2 points) For the reaction shown above in Problem 1 Part a, write the rate law and give the units of the rate constant if the reaction is, a) Following an elementary rate law, b) second order in A and overall second order, c) zero order in A and B, and d) second order in B and overall zero order
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


