Question: Please solve Write equilibrium (mass action) expressions for each of the following reactions. If either the numerator or denominator is 1, please enter 1. For
Please solve 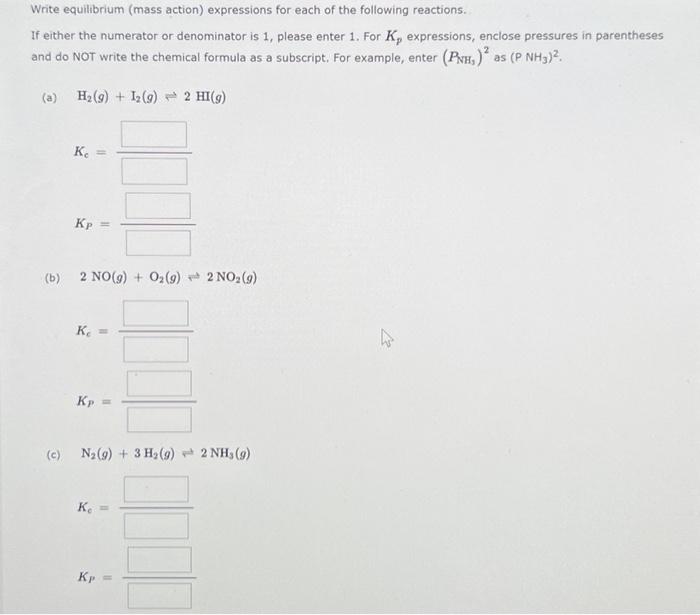
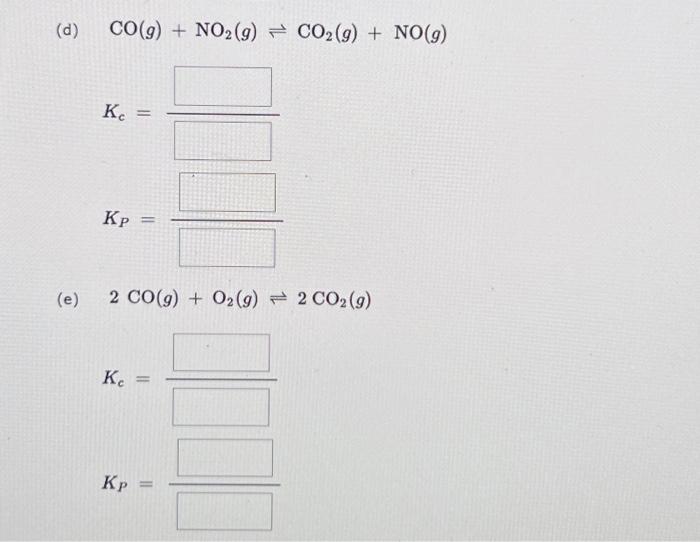


Write equilibrium (mass action) expressions for each of the following reactions. If either the numerator or denominator is 1, please enter 1. For K, expressions, enclose pressures in parentheses and do NOT write the chemical formula as a subscript. For example, enter (PNH) as (P NH3). (a) H(g) + 12(g) 2 HI(g) Ke= Kp (b) 2 NO(g) + O(9) 2 NO (9) K = W (c) N(g) + 3H(g) 2 NH (9) Ke Kp U (d) (e) CO(g) + NO2(g) CO2(g) + NO(g) Kc = Kp = 2 CO(g) + O2(g) = 2 CO2(g) Ke = Kp =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


