Question: please state the correct answer 07 Question (8 points) a Sec pare 570 Freezing point depression is a colligative property. 1st attempt MSec Periodic Table
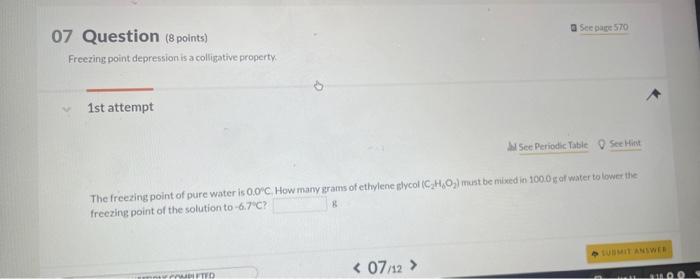
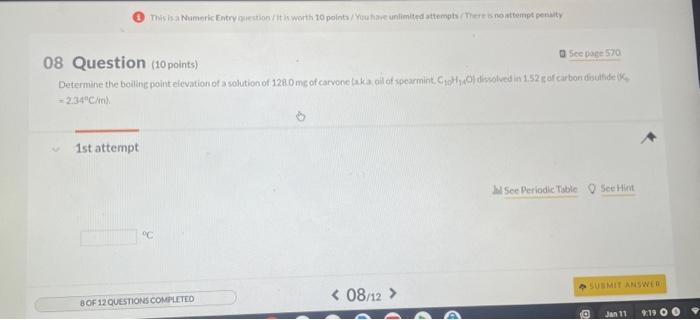
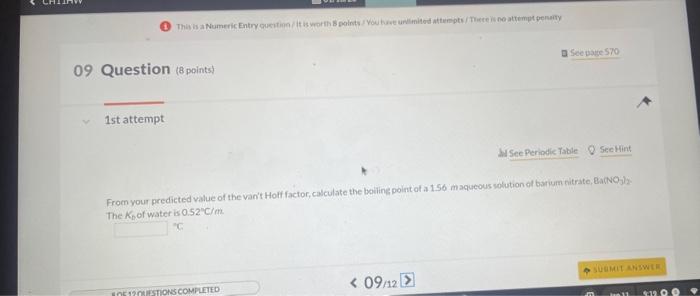
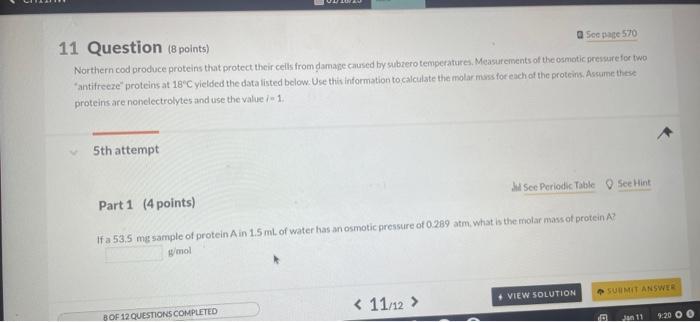
07 Question (8 points) a Sec pare 570 Freezing point depression is a colligative property. 1st attempt MSec Periodic Table 8 Siective The freezing point of pure water is 0.00C How many grams of ethylene glycol (C2H6O2) must be mixedin 100.0 af water to lower the freezing point of the solution to 6.7C ? 8. (1) Tossha Numpric Entry guestion / it is worth 20 points/You have unlimited attempts / Teres no attempt penaly a 5 ec poge 570 8 Question (10points) =2.34ACCinh 1st attempt From your predieted value of the van't Holf factor, calculate the boling point of a 1.56 maqueous solution af bariumnitrate, BadNOjl. The Khol of ater is 0.52C/m 'C. 1 Question (8 points) Northern cod produce proteins that protect their cells from damase caused by subzerotemperatures. Measureinents of the osmotic pressure fox two "antifrecee" proteins at 18C yielded the data listed below. Use this information to calculate the molar muss for each of the proteins, Assume these proteins are nonelectrolytes and use the value in 1. 5 th attempt Part 1 (4 points) Whee Periodic Table Q Seethint If a 53.5me sample of protein A in 1.5mL of water has anosmotic pressure of 0.289atm. what is the molar mass of protein A ? g/mol If a 53.5 ingsample of protein A in 1.5m L of wster has an osnotic pressure of 02agatm, what h the molir mass of protein Al? Part 2 (4 points) Q Seetint If a 53.9 mut sample of protein B in 1.75mL of water has an osmotic pressure of 0218 atm, what is the molar mass of protein 8 ? ghool 4th attempt 3rd attempt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


