Question: please step by step and calculate it Question 2: Given experimental data of equilibrium total pressure at 60C for the vapour-liquid equilibria in methyl-ester and
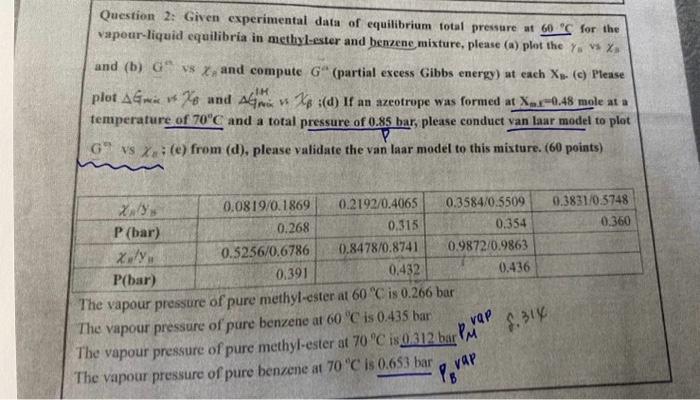
Question 2: Given experimental data of equilibrium total pressure at 60C for the vapour-liquid equilibria in methyl-ester and benzene mixture, please (a) plot the ys is xz and (b) Ga is h and compute Ga (partial excess Gibbs energy) at each Xn. (c) Please plot GmiswXB and GmisH$XB i(d) If an azeotrope was formed at Xex0.48 mole at a temperature of 70C and a total pressure of 0.85 har, please conduct van laar model to plot G9 is xa; (e) from (d), please validate the van laar model to this mixture. (60 points) The vapour pressure of pure methyl-ester at 00C.15 u. 200 pur The vapour pressure of pure benzene at 60C is 0.435 bar The vapour pressure of pure methyl-ester at 70C is 0.312 bar PM vaP M. 14 The vapour pressure of pure benzene at 70"C is 0.653 bar PBvaP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


