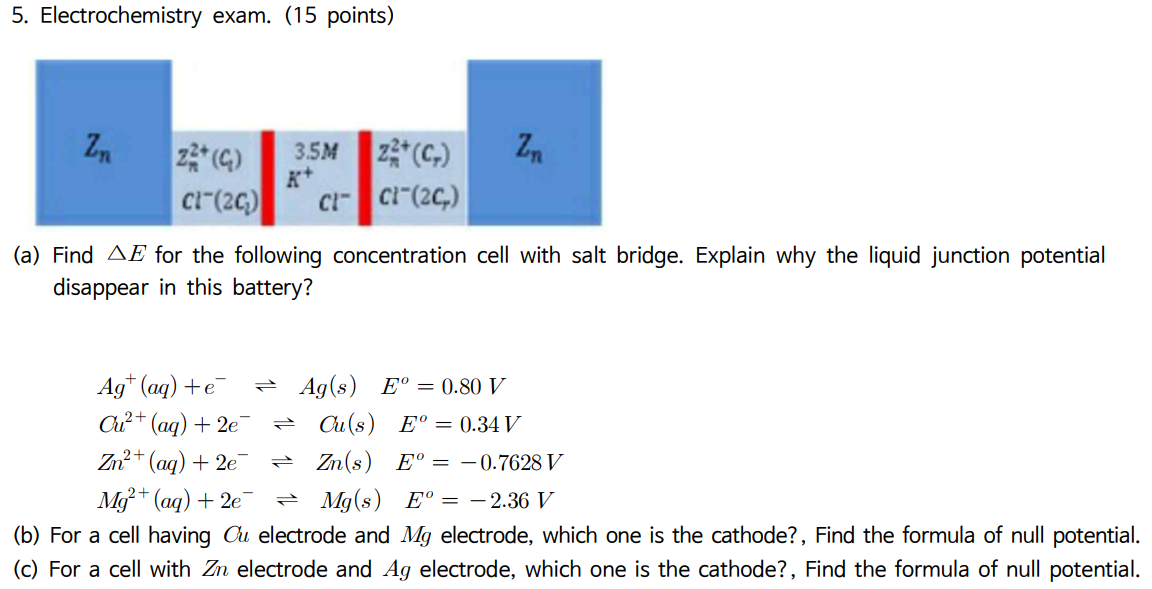
Question: Please the problem in detail. The subject I am learning is electrochemistry. 5. Electrochemistry exam. (15 points) (a) Find E for the following concentration cell
Please the problem in detail. The subject I am learning is electrochemistry.
5. Electrochemistry exam. (15 points) (a) Find E for the following concentration cell with salt bridge. Explain why the liquid junction potential disappear in this battery? Ag+(aq)+eAg(s)Eo=0.80VCu2+(aq)+2eP(s)Eo=0.34VZn2+(aq)+2en(s)Eo=0.7628VMg2+(aq)+2eMg(s)Eo=2.36V (b) For a cell having Cu electrode and Mg electrode, which one is the cathode?, Find the formula of null potential. (c) For a cell with Zn electrode and Ag electrode, which one is the cathode?, Find the formula of null potential. 5. Electrochemistry exam. (15 points) (a) Find E for the following concentration cell with salt bridge. Explain why the liquid junction potential disappear in this battery? Ag+(aq)+eAg(s)Eo=0.80VCu2+(aq)+2eP(s)Eo=0.34VZn2+(aq)+2en(s)Eo=0.7628VMg2+(aq)+2eMg(s)Eo=2.36V (b) For a cell having Cu electrode and Mg electrode, which one is the cathode?, Find the formula of null potential. (c) For a cell with Zn electrode and Ag electrode, which one is the cathode?, Find the formula of null potential
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


