Question: please try to do all please if not at least 2 but please its a request thank you Consider the reaction: C(s)+O2(g)CO2(g) Write the equilibrium

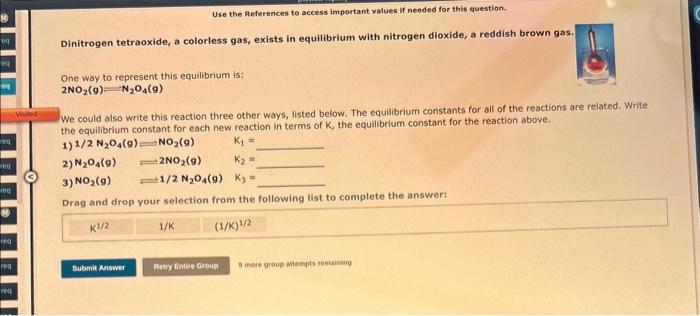
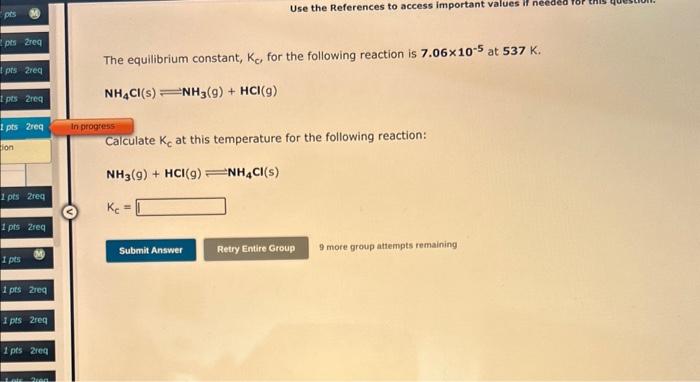

Consider the reaction: C(s)+O2(g)CO2(g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K1 and K2, for the reactions below: C(s)+1/2O2(g)CO(g)K1CO(g)+1/2O2(g)CO2(g)K2 For answers with both a subscript and a superscript, enter the subscript first. For example, enter K12 if the first equilibrium constant should be squared. K= Dinitrogen tetraoxide, a colorless gas, exists in equilibrium with nitrogen dioxide, a reddish brown gas. One way to represent this equilibrium is: 2NO2(g)=N2O4(g) We could also write this reaction three other ways, listed below. The equlibrium constants for all of the reactions are related. Write. the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above. 1) 1/2N2O4(g)=NO2(g)K1= 2) N2O4(g)NO2(9)K2= 3) NO2(g)1/2N2O4(g)K3= Drag and drop your selection from the following list to complete the answer: The equilibrium constant, Kc, for the following reaction is 7.06105 at 537K. NH4Cl(s)NH3(g)+HCl(g) Calculate Kc at this temperature for the following reaction: NH3(g)+HCl(g)NH4Cl(s) Kc= 9 more graup attempts remaining H3PO4(aq)+H2OH3O+(aq)+H2PO4(aq) The equilibrium constant for a second reaction is 6.20108 : H2PO4(aq)+H2OH3O+(aq)+HPO42(aq) Use this information to determine the equilibrium constant for the reaction: H3PO4(aq)+2H2O2H3O+(aq)+HPO42(aq) K= 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


