Question: Please use mathcad Example 8-5 Multiple Gas-Phase Reactions in a PBR The following complex gas-phase reactions follow elementary rate laws (1) A + 2B-C -1=
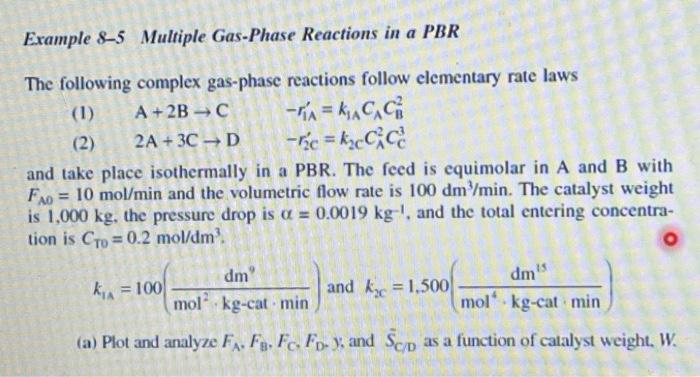
Example 8-5 Multiple Gas-Phase Reactions in a PBR The following complex gas-phase reactions follow elementary rate laws (1) A + 2B-C -1= kG (2) 2A + 3C D -rc = kccc and take place isothermally in a PBR. The feed is equimolar in A and B with = 10 mol/min and the volumetric flow rate is 100 dm /min. The catalyst weight is 1,000 kg, the pressure drop is a = 0.0019 kg-!. and the total entering concentra- tion is to = 0.2 mol/dm? FAO dm dm" kg = 100 and ky = 1,500 mol kg-cat min mol kg-cat min (a) Plot and analyze F. F. F. FD- y, and Scp as a function of catalyst weight, W
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


