Question: Please use OCTAVE /MATLAB to solve the above. 3. The progress of a homogeneous chemical reaction is followed and the rate constant and the order
 Please use OCTAVE /MATLAB to solve the above.
Please use OCTAVE /MATLAB to solve the above.
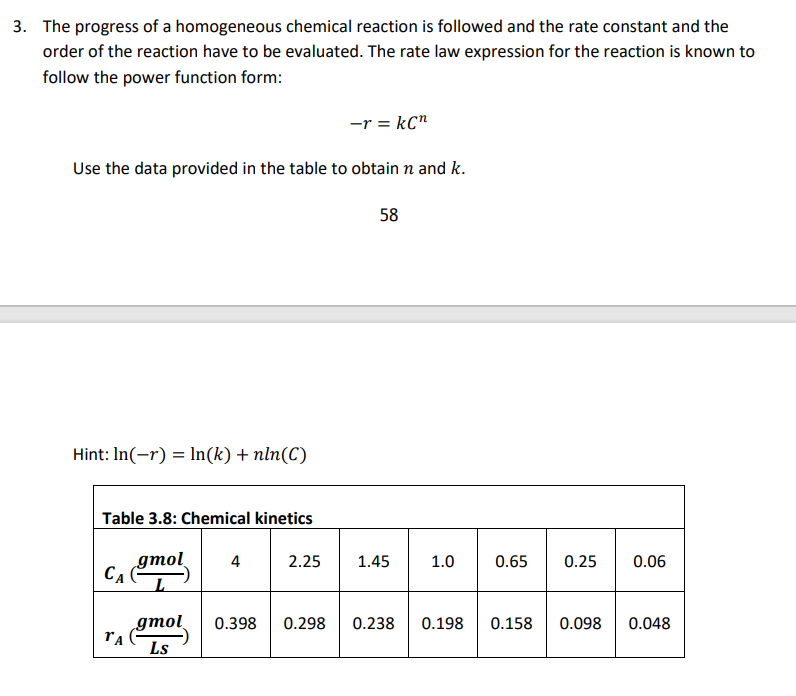
3. The progress of a homogeneous chemical reaction is followed and the rate constant and the order of the reaction have to be evaluated. The rate law expression for the reaction is known to follow the power function form: -r = kan Use the data provided in the table to obtain n and k. 58 Hint: In(-r) = ln(k) + nln(C) Table 3.8: Chemical kinetics gmol 4 2.25 1.45 1.0 0.65 0.25 0.06 ( 0.398 0.298 0.238 0.198 0.158 0.098 0.048 gmol Ls
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


