Question: please use options given in blue boxes to correct me. thanks Consider the balanced chemical reaction below. Calculate the percent yield for the reaction MnO,
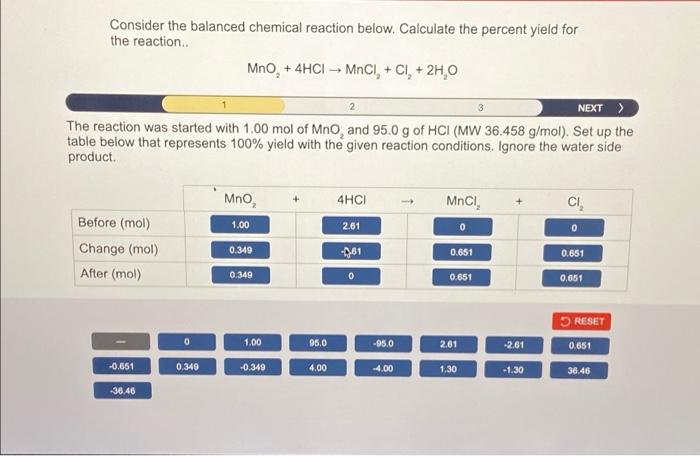
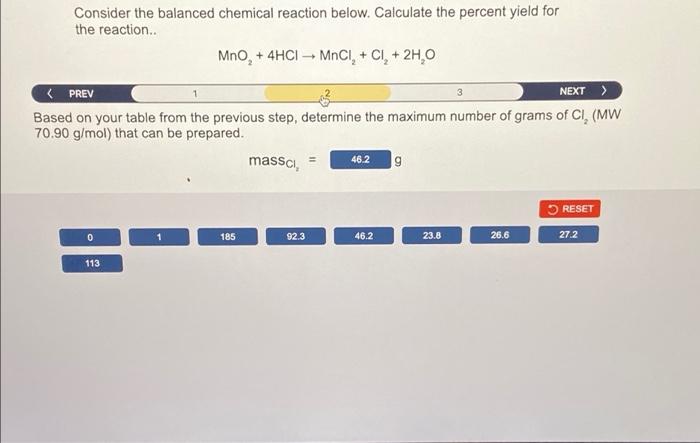
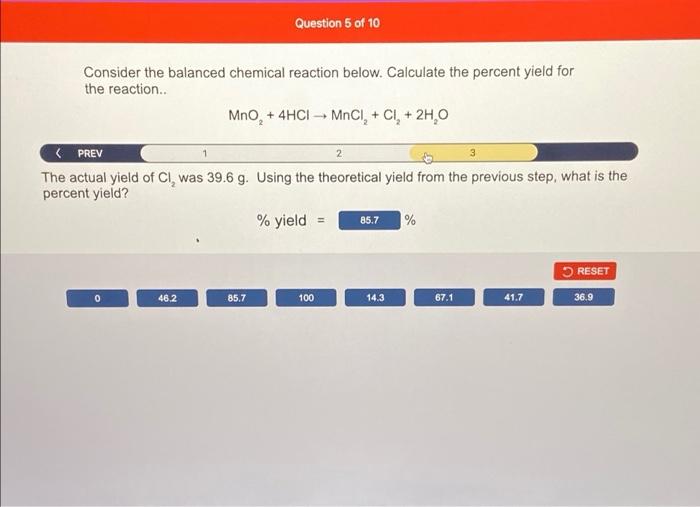
Consider the balanced chemical reaction below. Calculate the percent yield for the reaction MnO, + 4HCI - MOCI, + CI+ 2H,0 2 NEXT > The reaction was started with 1.00 mol of Mno, and 95.0 g of HCI (MW 36.458 g/mol). Set up the table below that represents 100% yield with the given reaction conditions. Ignore the water side product. Mno 4HCI MnCI, CI 1.00 2.61 0 0 Before (mol) Change (mol) After (mol) 0.349 -361 0.651 0.651 0.349 0 0.651 0.651 RESET 0.651 0 1,00 95.0 -95,0 2.61 -2.61 -0.651 0.349 -0,349 4.00 -4.00 1.30 -1.30 36.46 36.46 Consider the balanced chemical reaction below. Calculate the percent yield for the reaction.. MnO, + 4HCI MnCI+ CI+ 2H 0 + Based on your table from the previous step, determine the maximum number of grams of CI, (MW 70.90 g/mol) that can be prepared. massa 9 = 46.2 RESET 0 185 92.3 46.2 23.8 26.6 27.2 113 Question 5 of 10 Consider the balanced chemical reaction below. Calculate the percent yield for the reaction.. MnO, + 4HCI MNCI, + CI + 2H, O 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


