Question: PLEASE USE POLYMATH! PLEASE ANSWER THOROUGHLY!!! I WILL UPVOTE IF THE ATTEMPT IS GOOD! I WILL DOWNVOTE SPAM ANSWERS! The reaction A+BC is carried out
 PLEASE USE POLYMATH! PLEASE ANSWER THOROUGHLY!!! I WILL UPVOTE IF THE ATTEMPT IS GOOD! I WILL DOWNVOTE SPAM ANSWERS!
PLEASE USE POLYMATH! PLEASE ANSWER THOROUGHLY!!! I WILL UPVOTE IF THE ATTEMPT IS GOOD! I WILL DOWNVOTE SPAM ANSWERS!
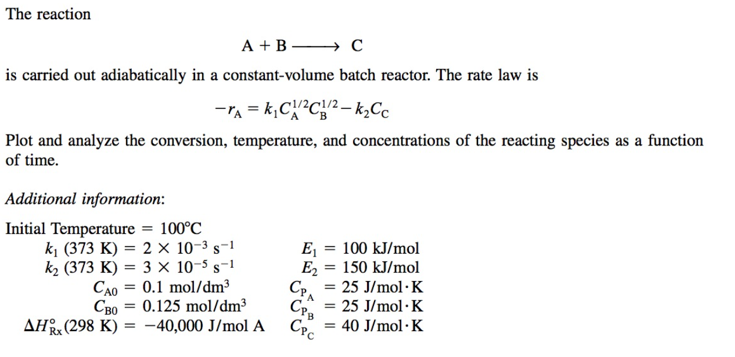
The reaction A+BC is carried out adiabatically in a constant-volume batch reactor. The rate law is rA=k1CA1/2CB1/2k2CC Plot and analyze the conversion, temperature, and concentrations of the reacting species as a function of time. Additional information: Initial Temperature =100C k1(373K)=2103s1E1=100kJ/molk2(373K)=3105s1E2=150kJ/molCA0=0.1mol/dm3CPA=25J/molKCB0=0.125mol/dm3CPBA=25J/molKHRx(298K)=40,000J/molACPC=40J/molK
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


