Question: Please use the numbers given in the question. If correct, I will upvote. Ethanol at 25 C and 24.0% excess air at 25 C enter
Please use the numbers given in the question. If correct, I will upvote.
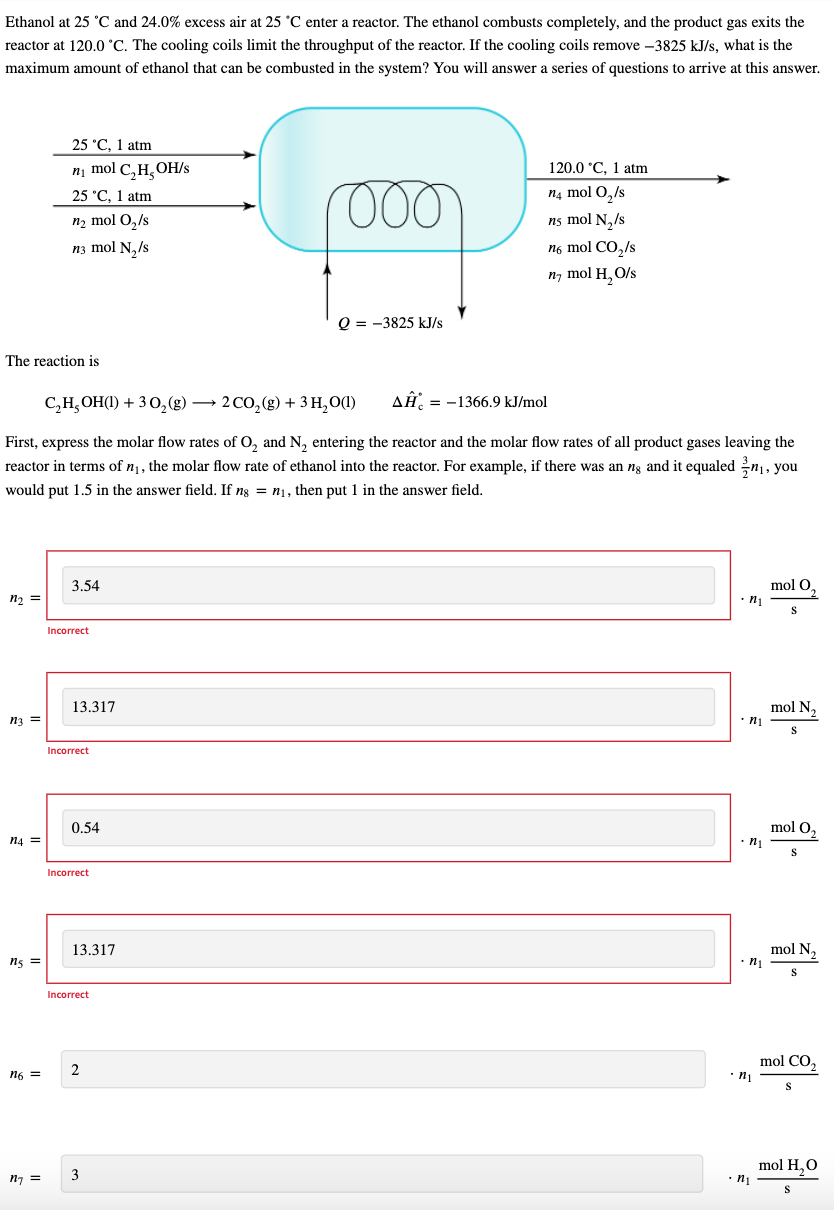
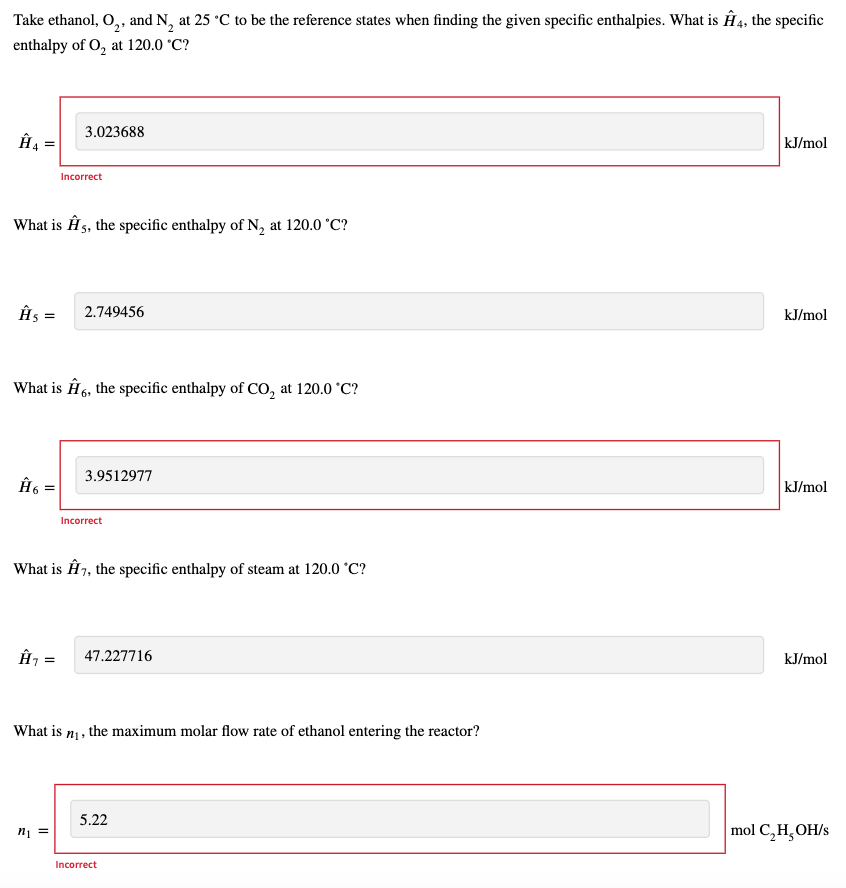
Ethanol at 25 C and 24.0% excess air at 25 C enter a reactor. The ethanol combusts completely, and the product gas exits the reactor at 120.0 C. The cooling coils limit the throughput of the reactor. If the cooling coils remove -3825 kJ/s, what is the maximum amount of ethanol that can be combusted in the system? You will answer a series of questions to arrive at this answer. 25 C, 1 atm ni mol C,H,OH/S 25 'C, 1 atm n2 mol O2/S n3 mol N / 000 120.0 C, 1 atm ' n4 mol 0,/s ns mol N /s no mol CO2/s n, mol H, Ols Q = -3825 kJ/s The reaction is C,H,OH(1) + 302(g) 2002 (8) + 3 H, 0(1) CO2g A = -1366.9 kJ/mol = = First, express the molar flow rates of O, and N, entering the reactor and the molar flow rates of all product gases leaving the reactor in terms of n, the molar flow rate of ethanol into the reactor. For example, if there was an ng and it equaled {n, you would put 1.5 in the answer field. If ng = ni, then put 1 in the answer field. 3.54 mol O n2 = ni S Incorrect 13.317 N mol N n3 = ni S Incorrect 0.54 mol O2 n4 = .nu S Incorrect 13.317 mol N ng = ni S Incorrect mol CO, n6 = 2 . S mol H, n = 3 .n1 S Take ethanol, O2, and N, at 25 C to be the reference states when finding the given specific enthalpies. What is 4, the specific enthalpy of Oat 120.0 C? 3.023688 4 = kJ/mol Incorrect What is A5, the specific enthalpy of N, at 120.0 C? s = 2.749456 kJ/mol What is A6, the specific enthalpy of Co, at 120.0C? Q = 3.9512977 kJ/mol Incorrect What is A7, the specific enthalpy of steam at 120.0 C? 1 = 47.227716 kJ/mol What is ni, the maximum molar flow rate of ethanol entering the reactor? 5.22 n = mol C, H, OH/s Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


