Question: Please use the Red and Blue boxes to help me organize the equation and fill in the blanks. I dont just need the anwser. Will
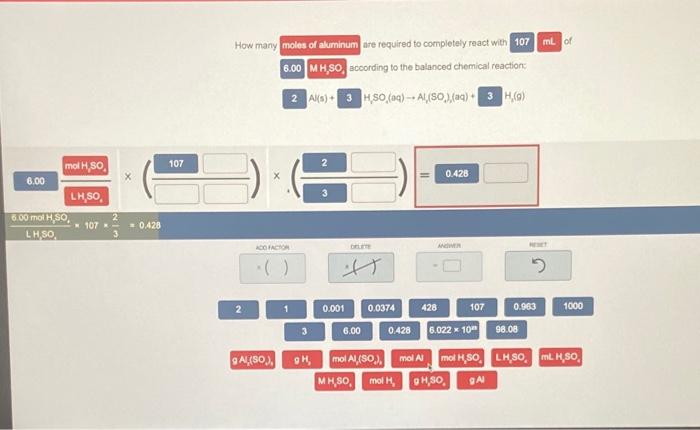
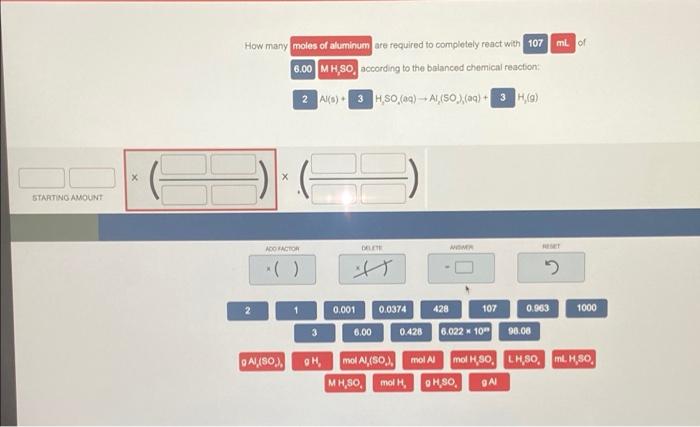
mL of How many moles of aluminum are required to completely react with 107 6.00 MH,SO, according to the balanced chemical reaction 2 Alls) 3 H,SO (aq) + Al(80) (aq) + 3 H) 107 11 0.428 mol H, SO 6.00 LHSO. 6.00 mol/S0 * 107 LHSO = 0.428 TACHOR 2 1 0.001 0.0374 428 107 0.963 1000 3 6.00 0.420 6.022 x 10 98.08 9 Al(SO). GH mol Al,(SO.. mol Al mol H,SO, LHSO mH SO, MH,SO mol, OH,SO GA ml of How many moles of aluminum are required to completely react with 107 6.00 MH,SO, according to the balanced chemical reaction 2Al(s) 3 HSO (0) -- A1,(50),(g) + 3HO) X STARTINO AMOUNT ADO FACTOR DELETE WOMIR 1 0.001 0.0374 428 107 0.963 1000 3 6.00 0.428 6.022 10 96.08 Al(80.). OH mol A,(50%, MH,SO mol mol Al mol H,80, LHSO, MH,80, OH,SO QA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


