Question: please work out on paper DO NOT ANSWER THIS PROBLEM WITH A CODE For aerobic fermentation, calculate the oxygen mass-transfer rate (mol/s) from each 100m-diameter
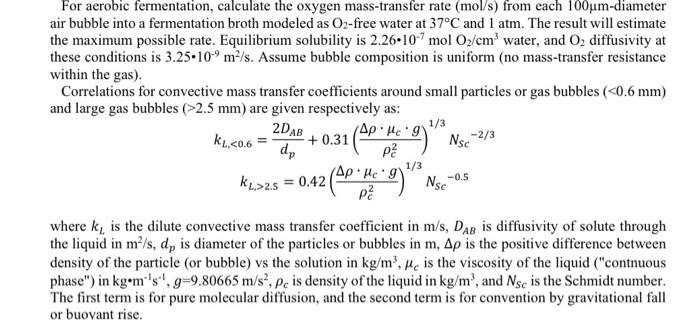
For aerobic fermentation, calculate the oxygen mass-transfer rate (mol/s) from each 100m-diameter air bubble into a fermentation broth modeled as O2-free water at 37C and 1atm. The result will estimate the maximum possible rate. Equilibrium solubility is 2.26107molO2/cm3 water, and O2 diffusivity at these conditions is 3.25109m2/s. Assume bubble composition is uniform (no mass-transfer resistance within the gas). Correlations for convective mass transfer coefficients around small particles or gas bubbles (2.5mm) are given respectively as: kL,2.5=0.42(c2cg)1/3NSc0.5 where kL is the dilute convective mass transfer coefficient in m/s,DAB is diffusivity of solute through the liquid in m2/s,dp is diameter of the particles or bubbles in m, is the positive difference between density of the particle (or bubble) vs the solution in kg/m3,c is the viscosity of the liquid ("contnuous phase") in kgm1s1,g=9.80665m/s2,c is density of the liquid in kg/m3, and NSc is the Schmidt number. The first term is for pure molecular diffusion, and the second term is for convention by gravitational fall or buoyant rise. For aerobic fermentation, calculate the oxygen mass-transfer rate (mol/s) from each 100m-diameter air bubble into a fermentation broth modeled as O2-free water at 37C and 1atm. The result will estimate the maximum possible rate. Equilibrium solubility is 2.26107molO2/cm3 water, and O2 diffusivity at these conditions is 3.25109m2/s. Assume bubble composition is uniform (no mass-transfer resistance within the gas). Correlations for convective mass transfer coefficients around small particles or gas bubbles (2.5mm) are given respectively as: kL,2.5=0.42(c2cg)1/3NSc0.5 where kL is the dilute convective mass transfer coefficient in m/s,DAB is diffusivity of solute through the liquid in m2/s,dp is diameter of the particles or bubbles in m, is the positive difference between density of the particle (or bubble) vs the solution in kg/m3,c is the viscosity of the liquid ("contnuous phase") in kgm1s1,g=9.80665m/s2,c is density of the liquid in kg/m3, and NSc is the Schmidt number. The first term is for pure molecular diffusion, and the second term is for convention by gravitational fall or buoyant rise
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


