Question: Please work out the problem from scratch. A solution has already been posted for this question before on Chegg, but it is very incorrect. Please
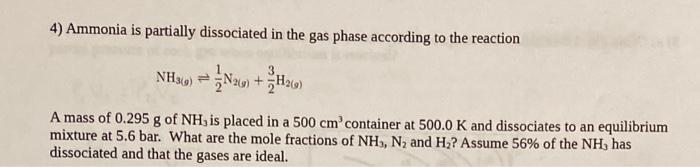
4) Ammonia is partially dissociated in the gas phase according to the reaction NH3(g)21N2(g)+23H2(g) A mass of 0.295g of NH3 is placed in a 500cm3 container at 500.0K and dissociates to an equilibrium mixture at 5.6 bar. What are the mole fractions of NH3,N2 and H2 ? Assume 56% of the NH3 has dissociated and that the gases are ideal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


