Question: please work these 3 questions for me 9. Complete the table below (10 marks) Concentration of Temperature Electrode Gibbs Free Temperature Entropy Enthalpy Cu ZN
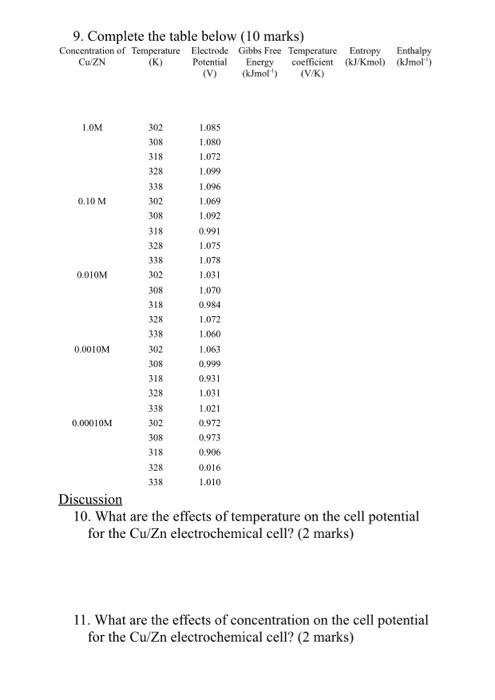
9. Complete the table below (10 marks) Concentration of Temperature Electrode Gibbs Free Temperature Entropy Enthalpy Cu ZN (K) Potential Energy coefficient (kJ/kmol) (kJmol) (V) (kJmol' (VK) 1.OM 302 308 318 328 338 302 1.ORS 1.080 1.072 1.099 1.096 1.069 0.10 M 308 1.092 318 328 338 302 0.010M 308 0.0010M 318 328 338 302 308 318 328 338 302 0.991 1.075 1.078 1.031 1.070 0.984 1.072 1.060 1.063 0.999 0.931 1.031 1.021 0.972 0.973 0.906 0.016 1.010 0.00010M 308 318 328 338 Discussion 10. What are the effects of temperature on the cell potential for the Cu/Zn electrochemical cell? (2 marks) 11. What are the effects of concentration on the cell potential for the Cu Zn electrochemical cell? (2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


