Question: please write a hypothesis for this lab using the expiremental questions below. Lxperiment 1: Which radical halogenation is more selective? An investigation into radical chlorination
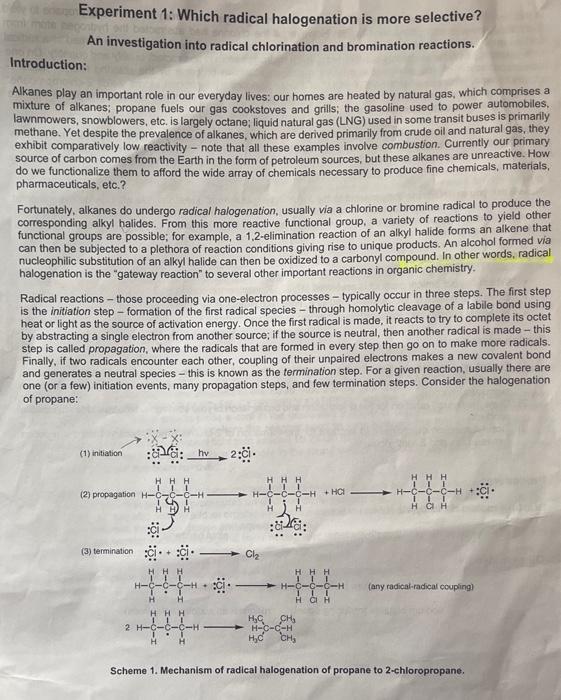

Lxperiment 1: Which radical halogenation is more selective? An investigation into radical chlorination and bromination reactions. Introduction: Alkanes play an important role in our everyday lives: our homes are heated by natural gas, which comprises a mixture of alkanes; propane fuels our gas cookstoves and grills; the gasoline used to power automobiles. lawnmowers, snowblowers, etc. is largely octane; liquid natural gas (LNG) used in some transit buses is primarly methane. Yet despite the prevalence of alkanes, which are derived primarily from crude oil and natural gas, they exhibit comparatively low reactivity - note that all these examples involve combustion. Currently our primary source of carbon comes from the Earth in the form of petroleum sources, but these alkanes are unreactive. How do we functionalize them to afford the wide array of chemicals necessary to produce fine chemicals, materials. pharmaceuticals, etc.? Fortunately, alkanes do undergo radical halogenation, usually via a chlorine or bromine radical to produce the corresponding alkyl halides. From this more reactive functional group, a variety of reactions to yield other functional groups are possible; for example, a 1,2-elimination reaction of an alkyl halide forms an alkene that can then be subjected to a plethora of reaction conditions giving rise to unique products. An alcohol formed via nucleophilic substitution of an alkyl halide can then be oxidized to a carbonyl compound. In other words, radical halogenation is the "gateway reaction" to several other important reactions in organic chemistry. Radical reactions - those proceeding via one-electron processes - typically occur in three steps. The first step is the initiation step - formation of the first radical species - through homolytic cleavage of a labile bond using heat or light as the source of activation energy. Once the first radical is made, it reacts to try to complete its octet by abstracting a single electron from another source: if the source is neutral, then another radical is made - this step is called propagation, where the radicals that are formed in every step then go on to make more radicals. Finally, if two radicals encounter each other, coupling of their unpaired electrons makes a new covalent bond and generates a neutral species - this is known as the termination step. For a given reaction, usually there are one (or a few) initation events, many propagation steps, and few termination steps. Consider the halogenation of propane: (2) propagation (3) termination :c+cCl2 (any radical-radical couping) Scheme 1. Mechanism of radical halogenation of propane to 2-chloropropane. Experimental Questions: - Which regiochemical product is preferred (1,2,3, resonance stabilized)? - Does chlorination or bromination lead to a more regioselective product? - Is radical substitution preferred on sp3 or sp2 hybridized carbons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


