Question: Please write clearly and show steps-formulas and descriptions. And dont want same answers for this question n here. i want different solution. 2. Natural gas
Please write clearly and show steps-formulas and descriptions. And dont want same answers for this question n here. i want different solution.
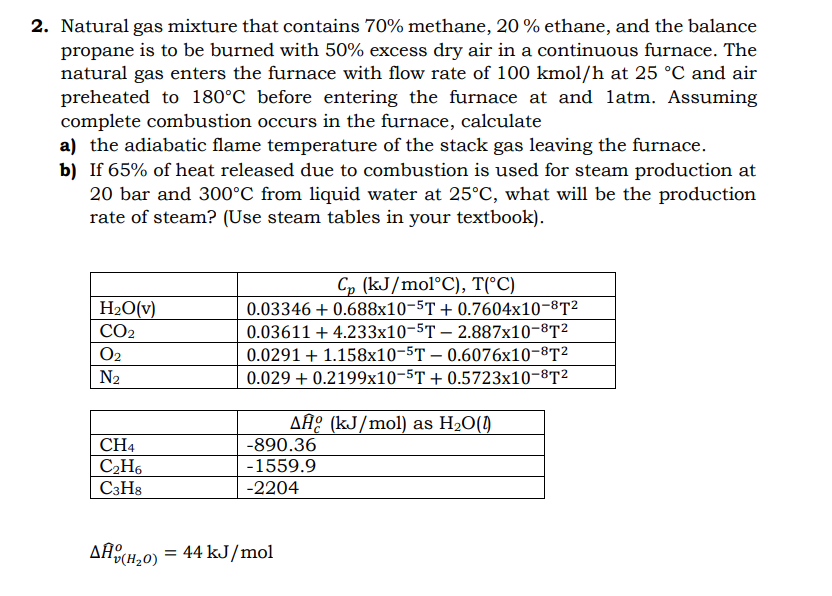
2. Natural gas mixture that contains 70% methane, 20% ethane, and the balance propane is to be burned with 50% excess dry air in a continuous furnace. The natural gas enters the furnace with flow rate of 100kmol/h at 25C and air preheated to 180C before entering the furnace at and 1 atm. Assuming complete combustion occurs in the furnace, calculate a) the adiabatic flame temperature of the stack gas leaving the furnace. b) If 65% of heat released due to combustion is used for steam production at 20 bar and 300C from liquid water at 25C, what will be the production rate of steam? (Use steam tables in your textbook). H^v(H2O)o=44kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


