Question: Please write clearly and show work. Given data below A) Calculate the solution of CORRECTION for the bottom rows marked IN GREEN. B) Calculate the
Please write clearly and show work.
Given data below
A) Calculate the solution of "CORRECTION" for the bottom rows marked IN GREEN.
B) Calculate the MEAN %KHP in unknown sample with ABSOLUTE and RELATIVE (ppt) 95% confidence intervals.
C) calculate the PROPOGATED ABSOLUTE and RELATIVE (ppt) UNCERTAINTIES IN the %KHP
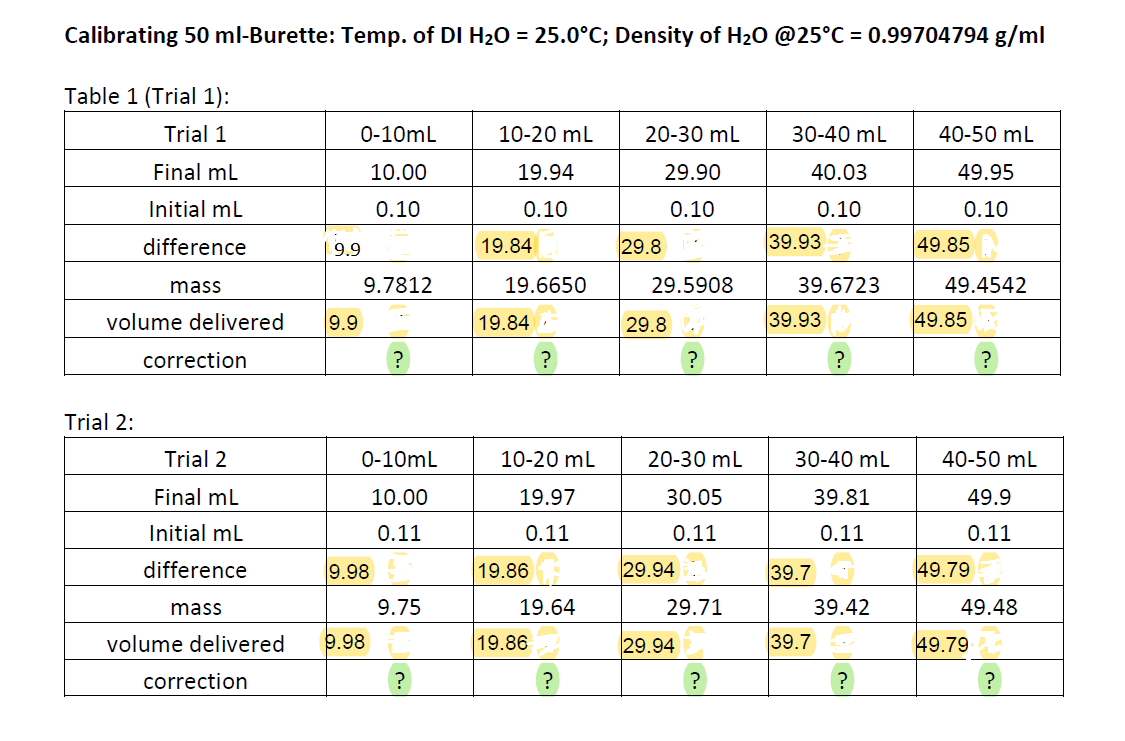
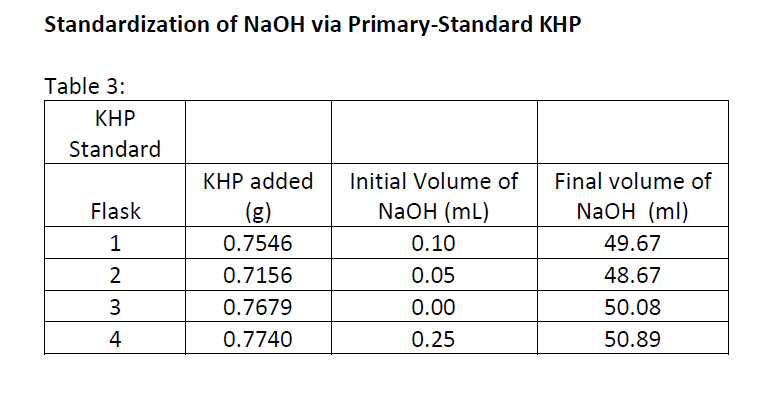
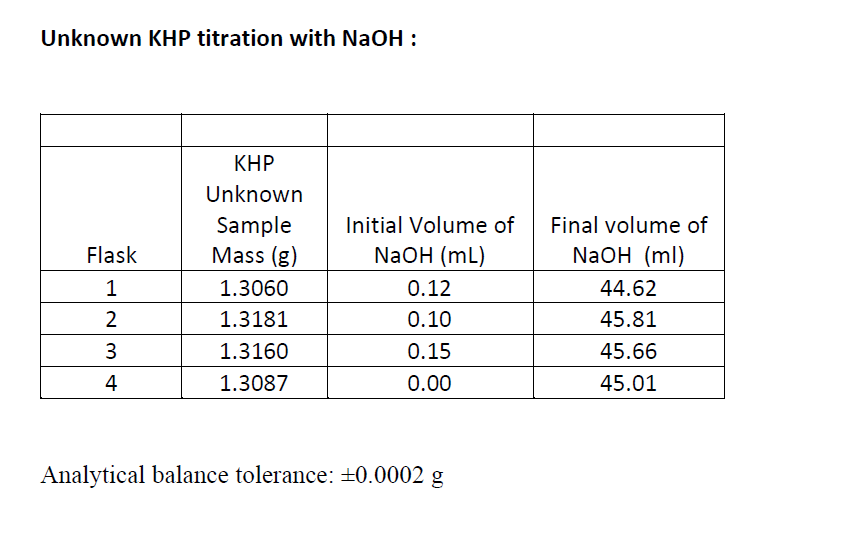
Calibrating 50 ml-Burette: Temp. of DI H20 = 25.0C; Density of H20 @25C = 0.99704794 g/ml Table 1 (Trial 1): Trial 1 0-10mL 10-20 mL 20-30 mL 30-40 mL 40-50 mL Final mL 10.00 19.94 29.90 40.03 49.95 Initial mL 0.10 0.10 0.10 0.10 0.10 39.93 difference 9.9 19.84 29.8 49.85 mass 9.7812 19.6650 29.5908 39.6723 49.4542 volume delivered 9.9 19.84 29.8 39.93 49.85 correction ? ? ? ? ? Trial 2: Trial 2 0-10mL 10-20 mL 20-30 mL 30-40 mL 40-50 mL 10.00 19.97 30.05 39.81 Final mL Initial mL 49.9 0.11 0.11 0.11 0.11 0.11 difference 9.98 19.86 29.94 39.7 49.79 mass 9.75 19.64 29.71 39.42 49.48 volume delivered 9.98 19.86 29.94 39.7 49.79 correction ? ? ? ? ? Standardization of NaOH via Primary-Standard KHP Table 3: KHP Standard KHP added Flask 1 Initial Volume of NaOH (mL) 0.10 0.05 0.00 0.7546 0.7156 0.7679 0.7740 Final volume of NaOH (ml) 49.67 48.67 50.08 50.89 2 3 4 0.25 Unknown KHP titration with NaOH : Flask 1 2 KHP Unknown Sample Mass (g) 1.3060 1.3181 1.3160 1.3087 Initial Volume of NaOH (mL) 0.12 0.10 0.15 Final volume of NaOH (ml) 44.62 45.81 45.66 45.01 3 4 0.00 Analytical balance tolerance: 0.0002 g Calibrating 50 ml-Burette: Temp. of DI H20 = 25.0C; Density of H20 @25C = 0.99704794 g/ml Table 1 (Trial 1): Trial 1 0-10mL 10-20 mL 20-30 mL 30-40 mL 40-50 mL Final mL 10.00 19.94 29.90 40.03 49.95 Initial mL 0.10 0.10 0.10 0.10 0.10 39.93 difference 9.9 19.84 29.8 49.85 mass 9.7812 19.6650 29.5908 39.6723 49.4542 volume delivered 9.9 19.84 29.8 39.93 49.85 correction ? ? ? ? ? Trial 2: Trial 2 0-10mL 10-20 mL 20-30 mL 30-40 mL 40-50 mL 10.00 19.97 30.05 39.81 Final mL Initial mL 49.9 0.11 0.11 0.11 0.11 0.11 difference 9.98 19.86 29.94 39.7 49.79 mass 9.75 19.64 29.71 39.42 49.48 volume delivered 9.98 19.86 29.94 39.7 49.79 correction ? ? ? ? ? Standardization of NaOH via Primary-Standard KHP Table 3: KHP Standard KHP added Flask 1 Initial Volume of NaOH (mL) 0.10 0.05 0.00 0.7546 0.7156 0.7679 0.7740 Final volume of NaOH (ml) 49.67 48.67 50.08 50.89 2 3 4 0.25 Unknown KHP titration with NaOH : Flask 1 2 KHP Unknown Sample Mass (g) 1.3060 1.3181 1.3160 1.3087 Initial Volume of NaOH (mL) 0.12 0.10 0.15 Final volume of NaOH (ml) 44.62 45.81 45.66 45.01 3 4 0.00 Analytical balance tolerance: 0.0002 g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


