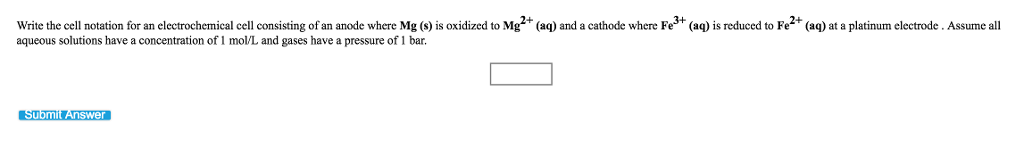
Question: please write clearly Write the cell notation for an electrochemical cell consisting of an anode where Mg (s) is oxidized to Mg2+ (aq) and a
 please write clearly
please write clearly
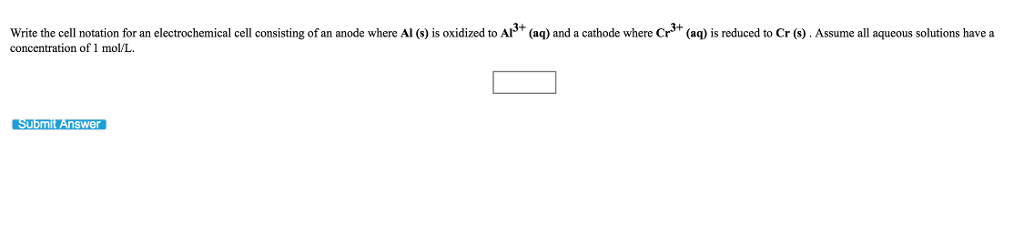
Write the cell notation for an electrochemical cell consisting of an anode where Mg (s) is oxidized to Mg2+ (aq) and a cathode where Fe+ (aq) is reduced to Fe+ (aq) at a platinum electrode. Assume all aqueous solutions have a concentration of 1 mol/L and gases have a pressure of 1 bar. Submit Answer 0 Write the cell notation for an electrochemical cell consisting of an anode where Al (s) is oxidized to Al+ (aq) and a cathode where Cr+ (aq) is reduced to Cr (s). Assume all aqueous solutions have a concentration of 1 mol/L. Submit Answer
Step by Step Solution
3.50 Rating (147 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


