Question: please write in clear handwriting , thank you!! Th Concentration Calculations AX attempt.php?attempt 1305585&cmid=2030 al Science 20 rses / Physical Science 20 / FC3 -
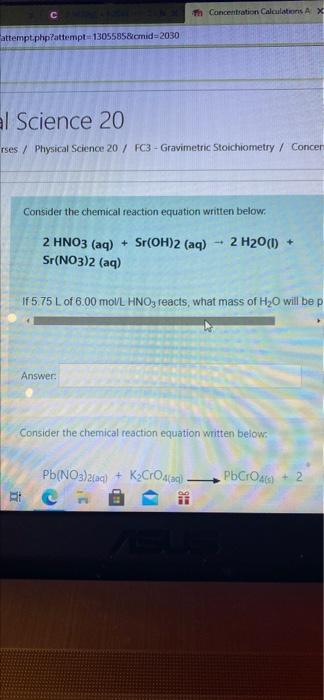
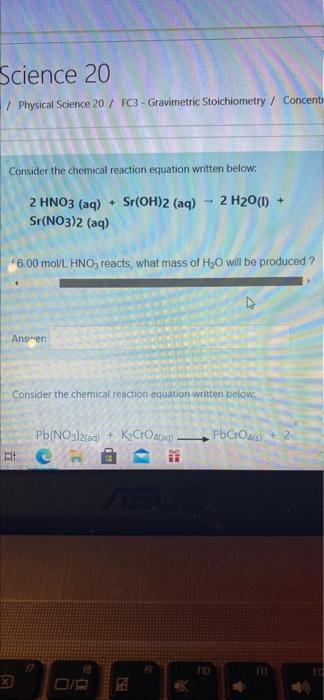
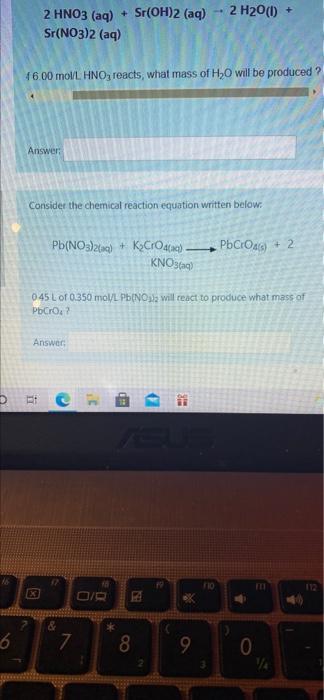
Th Concentration Calculations AX attempt.php?attempt 1305585&cmid=2030 al Science 20 rses / Physical Science 20 / FC3 - Gravimetric Stoichiometry / Concer Consider the chemical reaction equation written below. - 2 H2O(l) + 2 HNO3(aq) + Sr(OH)2 (aq) Sr(NO3)2 (aq) If575 L of 6.00 moVL HNO3 reacts, what mass of H2O will be p Answer: Consider the chemical reaction equation written below. Pb(NO3)2(aq) + Kardaq). PbCross) + 2 MI Science 20 / Physical Science 20 / FC3 - Gravimetric Stoichiometry / Concent Consider the chemical reaction equation written below: 2 HNO3(aq) + Sr(OH)2 (aq) Sr(NO3)2 (aq) 2 H2O(1) + *6.00 mol/L. HNO3 reacts, what mass of H2O will be produced ? Answer: Consider the chemical reaction equation written below: POCHOA Pb(NO3)2(aq) + KO) Etc S 2 H20() + 2 HNO3 (aq) + Sr(OH)2 (aq) Sr(NO3)2 (aq) 4600 mol/L. HNO, reacts, what mass of H2O will be produced ? Answer: Consider the chemical reaction equation written below: Pb(NO3)2(a) + KyCrO (q). KNO3(aq) PbCO) + 2 045 Lof 0.350 mol/L Pb(NO3 will react to produce what mass of PbCrO2 Answer: bic BIB M 8 D X 6 7 8 9 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


