Question: please write out all work!! x Check that you are using K. Check the vales of the coefficients in the poynomial. A sample of carbon
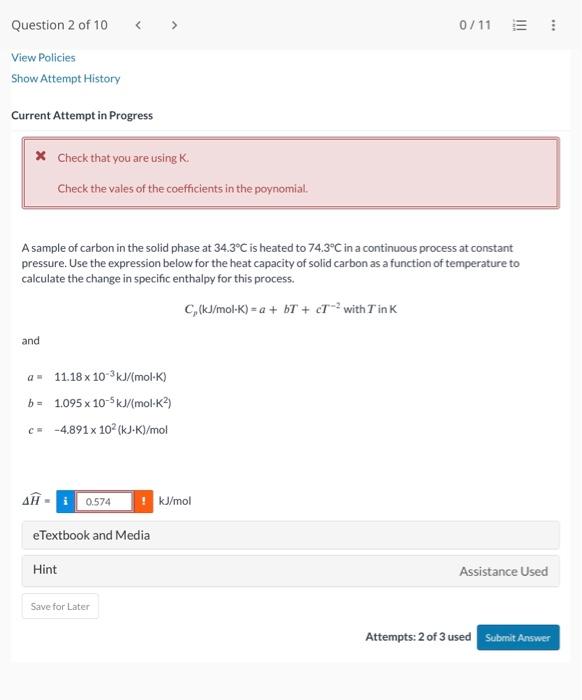
x Check that you are using K. Check the vales of the coefficients in the poynomial. A sample of carbon in the solid phase at 34.3C is heated to 74.3C in a continuous process at constant pressure. Use the expression below for the heat capacity of solid carbon as a function of temperature to calculate the change in specific enthalpy for this process. Cp(kJ/molK)=a+bT+cT2withTinK and a=11.18103kJ/(molK)b=1.095105kJ/(molK2)c=4.891102(kJK)/mol H= eTextbook and Media
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


