Question: Please write the letters, words and numbers clearly. The answer can be short. In addition, please answer all questions, and do not copy other people's
Please write the letters, words and numbers clearly. The answer can be short. In addition, please answer all questions, and do not copy other people's answers to me, I will give you a good evaluation based on the answer.
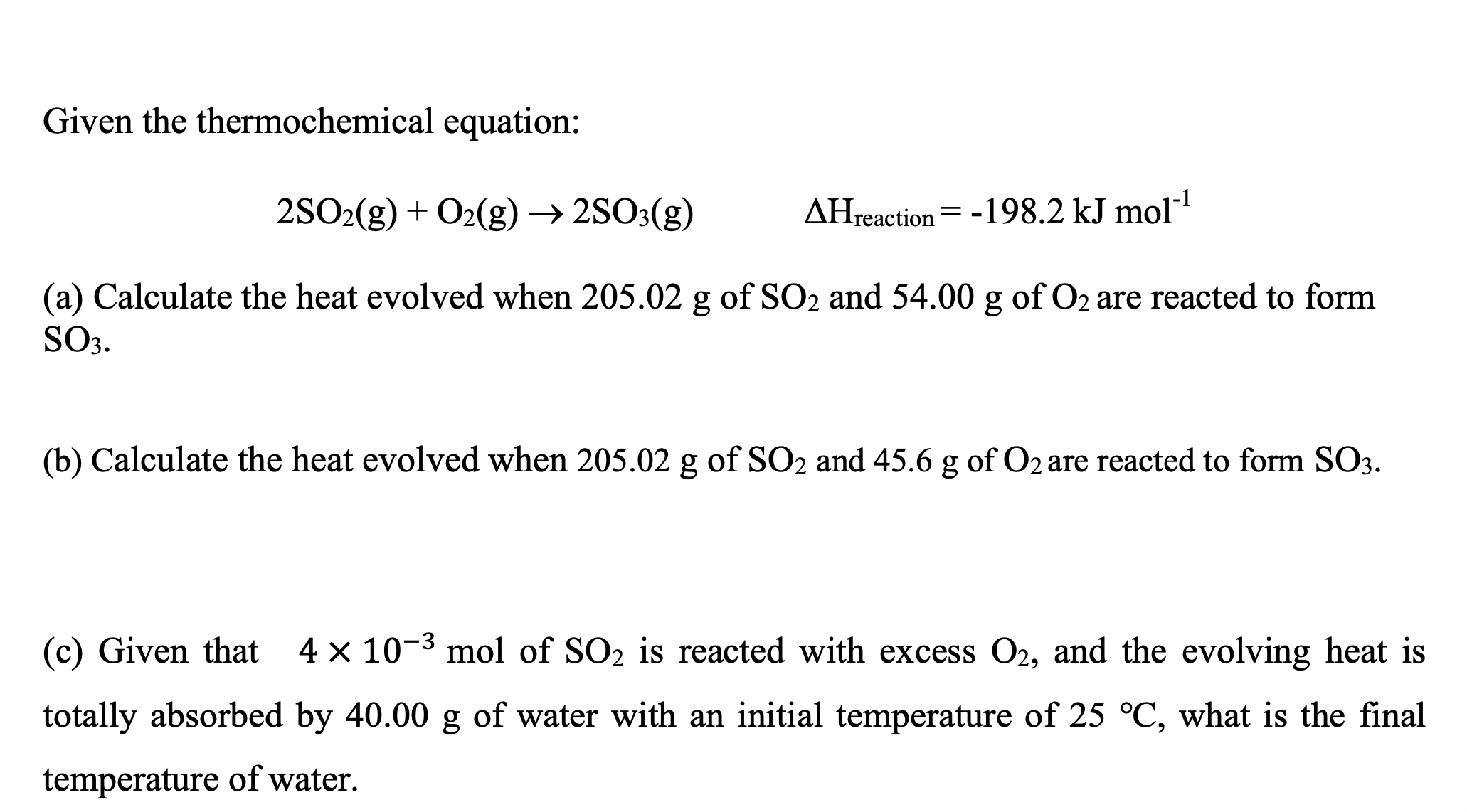
Given the thermochemical equation: 2SO2(g)+O2(g)2SO3(g)Hreaction=198.2kJmol1 (a) Calculate the heat evolved when 205.02g of SO2 and 54.00g of O2 are reacted to form SO3 (b) Calculate the heat evolved when 205.02g of SO2 and 45.6g of O2 are reacted to form SO3. (c) Given that 4103mol of SO2 is reacted with excess O2, and the evolving heat is totally absorbed by 40.00g of water with an initial temperature of 25C, what is the final temperature of water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


