Question: Please write the step by step process clearly, thank you! (Bonus) A quantity of ice at 0.0C is added to 64.3g of water in a
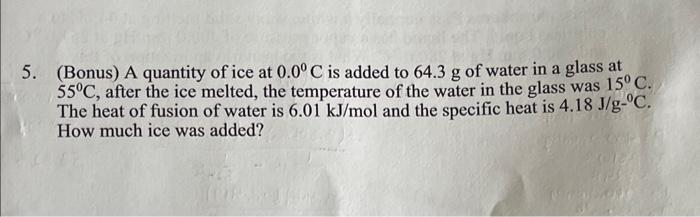
(Bonus) A quantity of ice at 0.0C is added to 64.3g of water in a glass at 55C, after the ice melted, the temperature of the water in the glass was 15C. The heat of fusion of water is 6.01kJ/mol and the specific heat is 4.18J/gC. How much ice was added
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


