Question: Please write the whole process and describe in detail . Thanks . The Langmuir adsorption model is written as below, in which [S-M) is the
Please write the whole process and describe in detail . Thanks .
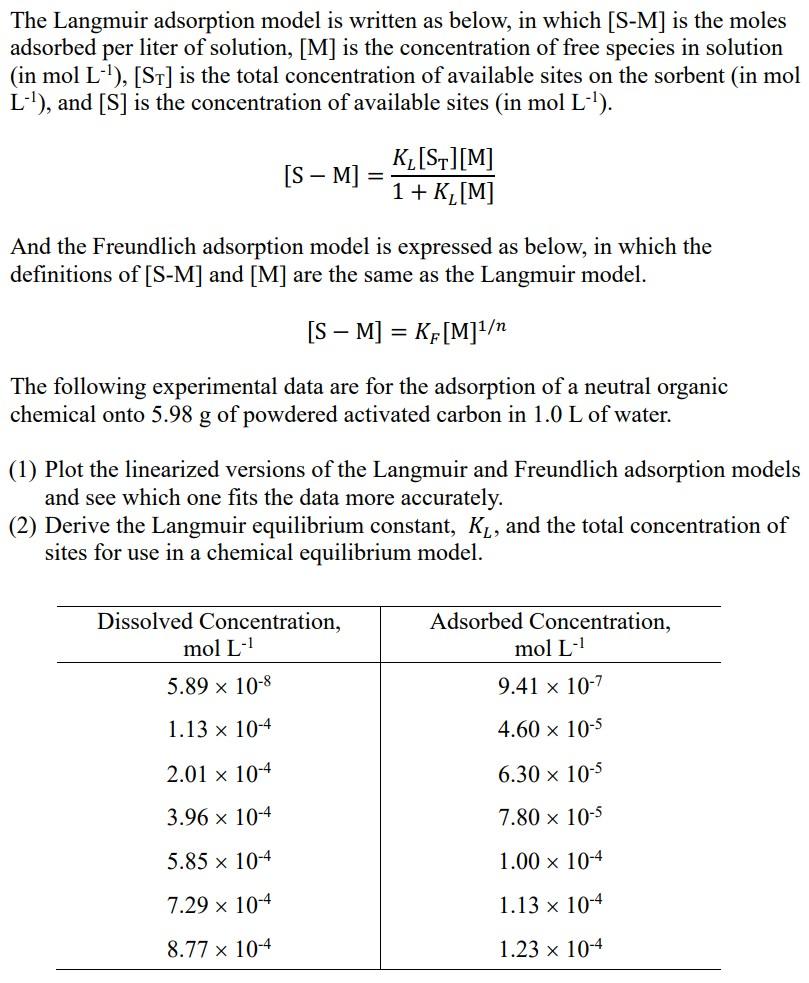
The Langmuir adsorption model is written as below, in which [S-M) is the moles adsorbed per liter of solution, [M] is the concentration of free species in solution (in mol L-'), [ST] is the total concentration of available sites on the sorbent (in mol L-1), and [S] is the concentration of available sites (in mol L-'). [S M] K_[S][M] 1 + K,[M] And the Freundlich adsorption model is expressed as below, in which the definitions of [S-M] and [M] are the same as the Langmuir model. [S M] = KF[M]1 = The following experimental data are for the adsorption of a neutral organic chemical onto 5.98 g of powdered activated carbon in 1.0 L of water. g (1) Plot the linearized versions of the Langmuir and Freundlich adsorption models and see which one fits the data more accurately. (2) Derive the Langmuir equilibrium constant, Ky, and the total concentration of sites for use in a chemical equilibrium model. Dissolved Concentration, mol L- Adsorbed Concentration, mol L- 9.41 x 10-7 5.89 x 10-8 1.13 x 10-4 4.60 x 10-5 2.01 x 10-4 6.30 x 10-5 3.96 x 10-4 7.80 x 10-5 5.85 x 10-4 1.00 x 10-4 7.29 x 10-4 1.13 x 10-4 8.77 x 10-4 1.23 x 10-4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


