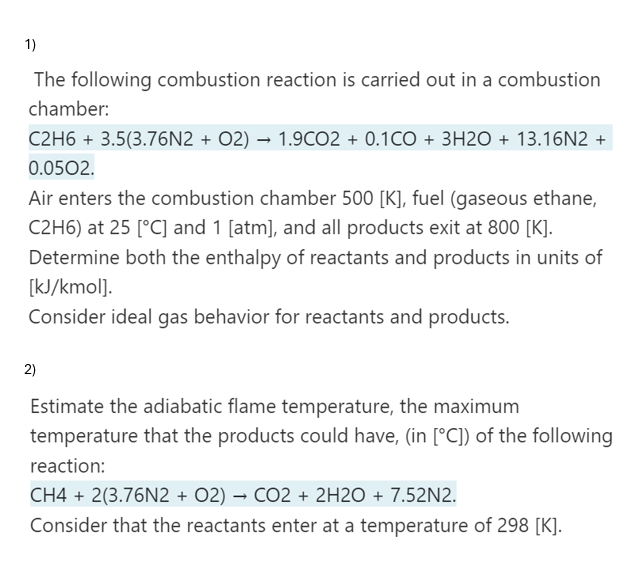
Question: Pleaseeeee help meee, take your timeeee 1 ) The following combustion reaction is carried out in a combustion chamber: C 2 H 6 + 3
Pleaseeeee help meee, take your timeeee
The following combustion reaction is carried out in a combustion
chamber:
Air enters the combustion chamber fuel gaseous ethane,
at and atm and all products exit at
Determine both the enthalpy of reactants and products in units of
kJkmol
Consider ideal gas behavior for reactants and products.
Estimate the adiabatic flame temperature, the maximum
temperature that the products could have, in of the following
reaction:
Consider that the reactants enter at a temperature of K
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


