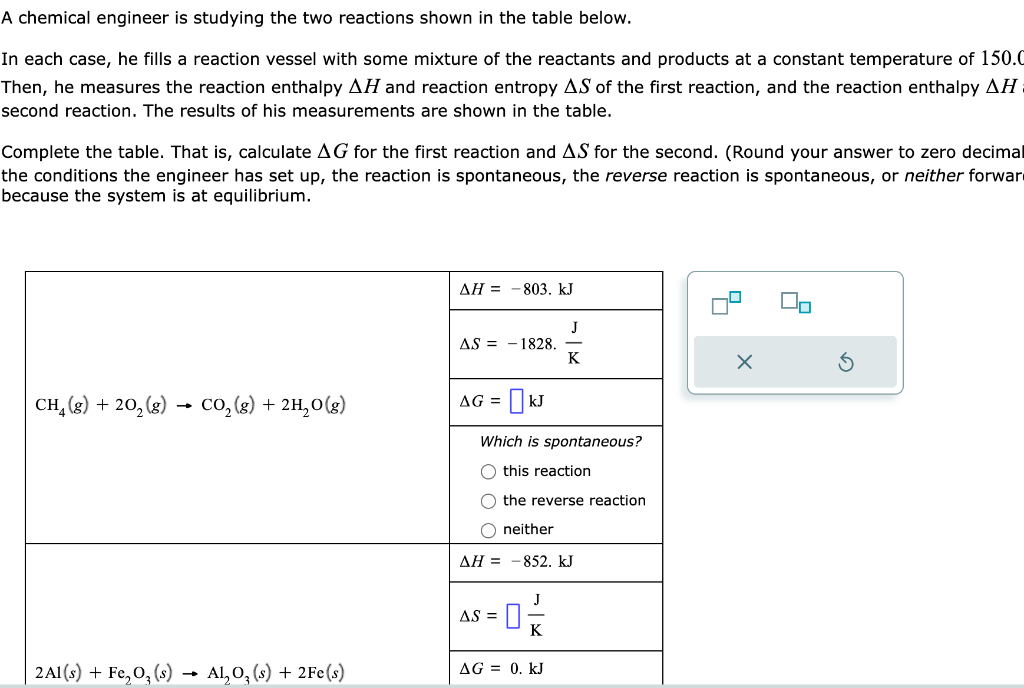
Question: PLEASEEEEEEEE A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture
 PLEASEEEEEEEE
PLEASEEEEEEEE
A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 150.0 Then, he measures the reaction enthalpy H and reaction entropy S of the first reaction, and the reaction enthalpy H second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate G for the first reaction and S for the second. (Round your answer to zero decimal the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forwar because the system is at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


