Question: Please,I want to different method solution for this can you by writing another solving? 1. Sphalerite is roasted at 750C and 1atm pressure in a
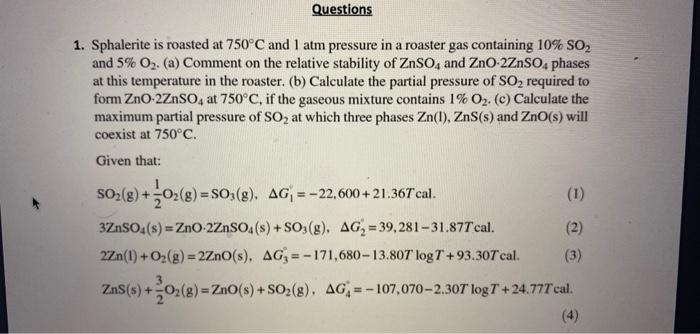
1. Sphalerite is roasted at 750C and 1atm pressure in a roaster gas containing 10%SO2 and 5%O2. (a) Comment on the relative stability of ZnSO4 and ZnO2ZnSO4 phases at this temperature in the roaster. (b) Calculate the partial pressure of SO2 required to form ZnO2ZnSO4 at 750C, if the gaseous mixture contains 1%O2. (c) Calculate the maximum partial pressure of SO2 at which three phases Zn(I),ZnS(s) and ZnO(s) will coexist at 750C. Given that: SO2(g)+21O2(g)=SO3(g),G1=22,600+21.36Tcal3ZnSO4(s)=ZnO2ZnSO4(s)+SO3(g),G2=39,28131.87Tcal2Zn(1)+O2(g)=2ZnO(s),G3=171,68013.80TlogT+93.30TcalZnS(s)+23O2(g)=ZnO(s)+SO2(g),G4=107,0702.30TlogT+24.77Tcal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


