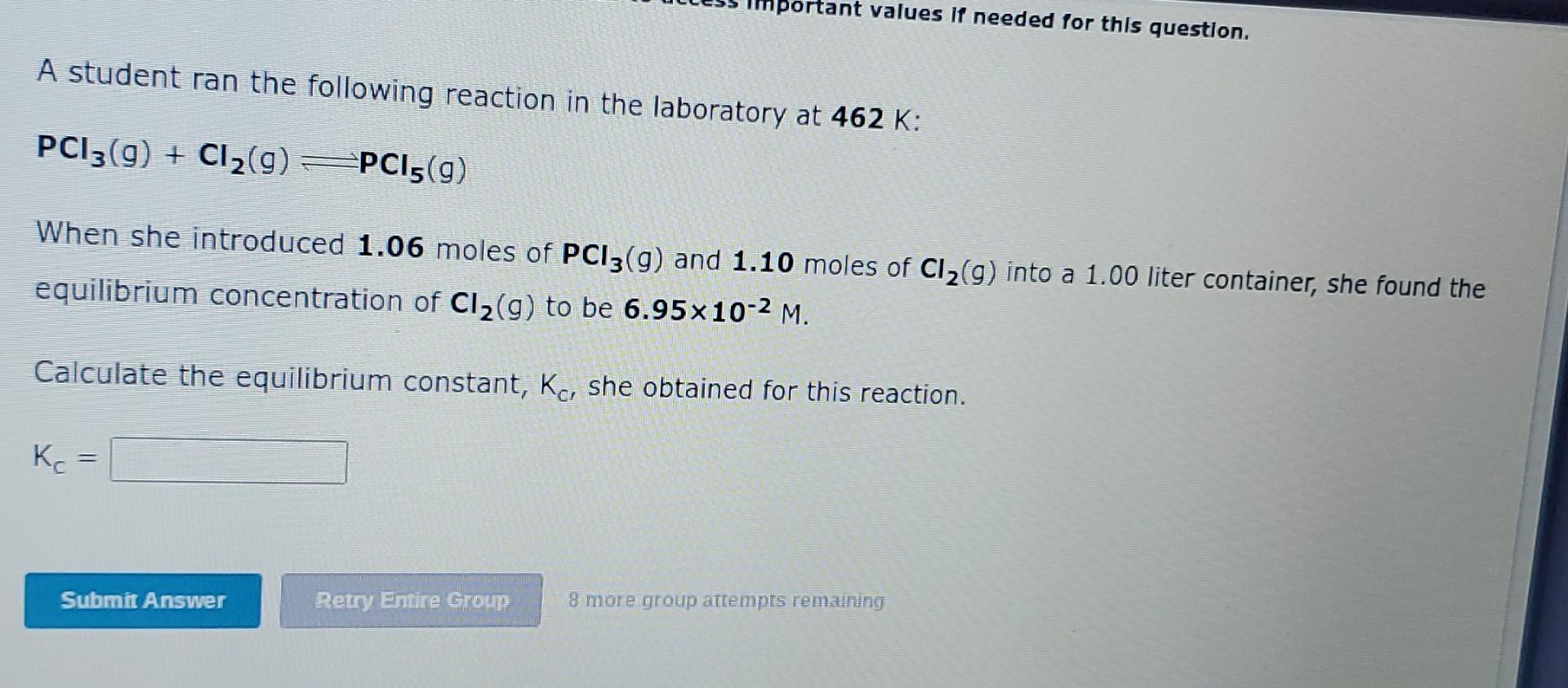
Question: pleasseee answer both. I keep getting them wrong! mportant values If needed for this question. A student ran the following reaction in the laboratory at
pleasseee answer both. I keep getting them wrong!
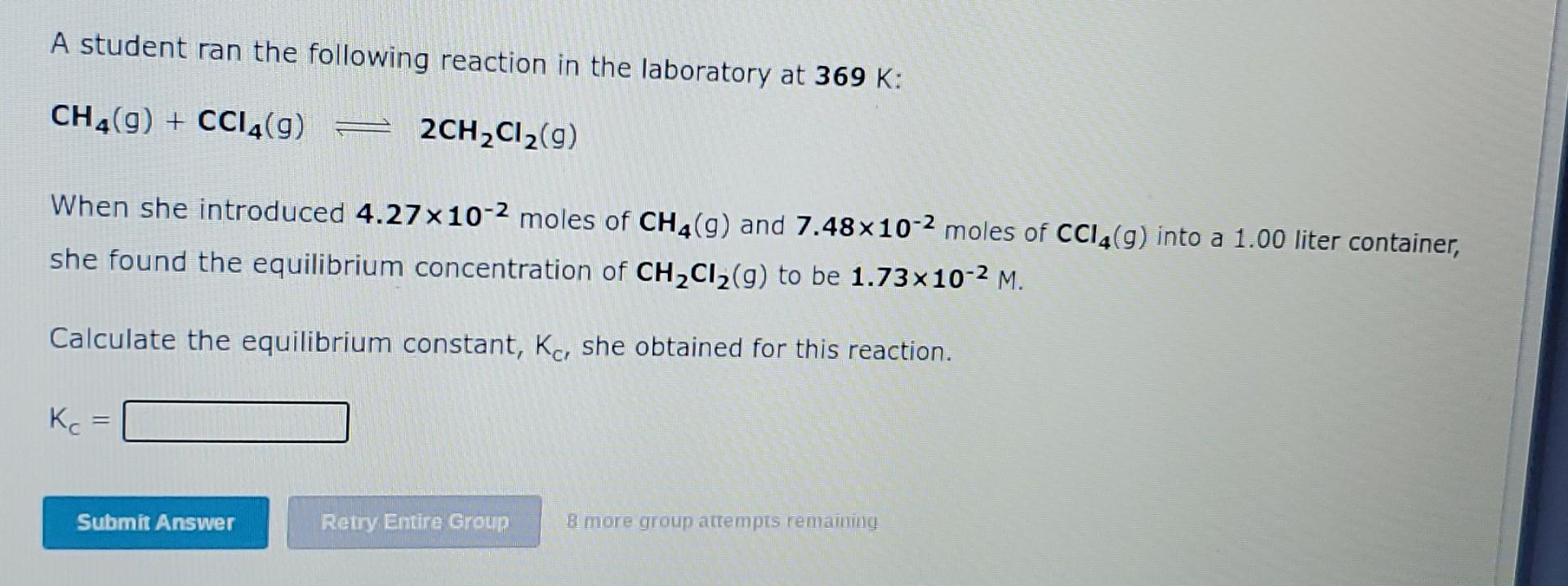
mportant values If needed for this question. A student ran the following reaction in the laboratory at 462 K: PCI3(9) + Cl2(9) PCI5(g) When she introduced 1.06 moles of PC13(9) and 1.10 moles of Cl2(9) into a 1.00 liter container, she found the equilibrium concentration of C12(g) to be 6.95x10-2 M. Calculate the equilibrium constant, Kc, she obtained for this reaction. K. Submit Answer Retry Entre Group 8 more group attempts remaining, A student ran the following reaction in the laboratory at 369 K: CH4(9) + CC14(g) = 2CH2C12(9) When she introduced 4.27x10-2 moles of CH4(9) and 7.48x10-2 moles of CCl4(9) into a 1.00 liter container, she found the equilibrium concentration of CH2C12(9) to be 1.73x10-2 M. Calculate the equilibrium constant, Kc, she obtained for this reaction. Kc = Submit Answer Retry Entire Group Bemore group attempts renvatting
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


