Question: PLEEASEEE HELP QUCKLYY pleasee !!!!! 2ASH(g) = 2As(s) + 3H(g) 2.13 g sample of pure AsH3(g) was placed in a sealed-empty 2.5 L flask and
PLEEASEEE HELP QUCKLYY pleasee !!!!!
2ASH(g) = 2As(s) + 3H(g) 2.13 g sample of pure AsH3(g) was placed in a sealed-empty 2.5 L flask and heated to 300C. When the equilibrium was attained, the total pressure in the flask was measured as 500 mmHg. (a) Calculate the initial pressure of AsH3(g) at 300C. (b) Calculate the equilibrium partial pressure of H2(g). (c) Calculate the equilibrium partial pressure of AsH3(g). (d) Calculate Kp for this reaction, if the partial pressures are expressed in atm. (e) Calculate Ke for the given reaction. (AsH3 = 77.95 gmol-, R = 0.0082 L atm/mol K, I atm = 760 mmHg)
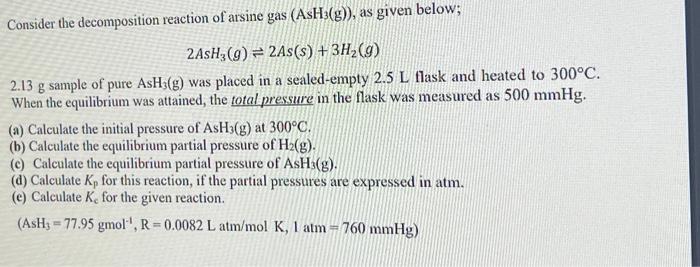
Consider the decomposition reaction of arsine gas (AsH3(g)), as given below; 2AsH3(g)2As(s)+3H2(g) 2.13g sample of pure AsH3(g) was placed in a sealed-empty 2.5L flask and heated to 300C. When the equilibrium was attained, the total pressure in the flask was measured as 500mmHg. (a) Calculate the initial pressure of AsH3(g) at 300C. (b) Calculate the equilibrium partial pressure of H2(g). (c) Calculate the equilibrium partial pressure of AsH3(g). (d) Calculate Kp for this reaction, if the partial pressures are expressed in atm. (c) Calculate Kc for the given reaction. (AsH3=77.95gmol1,R=0.0082Latm/molK,1atm=760mmHg)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


