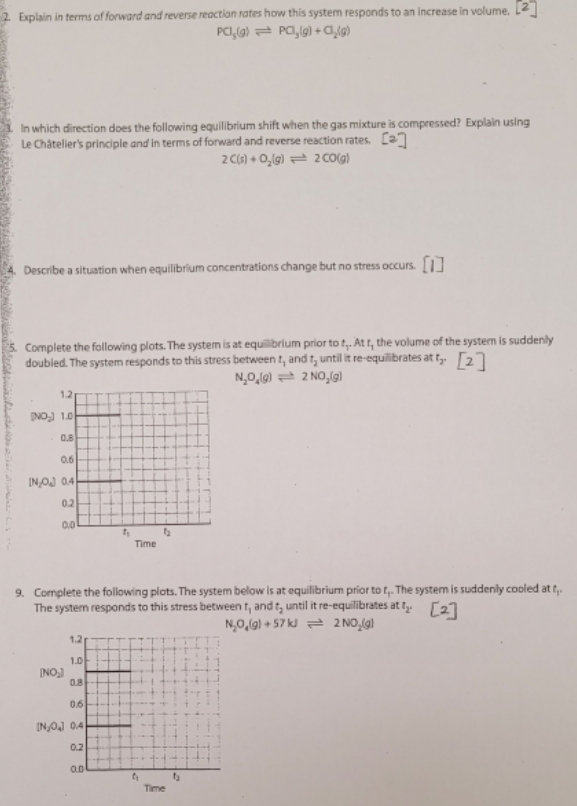
Question: plese do the two last ones 2. Explain in terms of forward and reverse reaction rates how this system responds to an increase in volume.
plese do the two last ones
2. Explain in terms of forward and reverse reaction rates how this system responds to an increase in volume. [2] PC),(a) PCI, (g) + (0) In which direction does the following equilibrium shift when the gas mixture is compressed? Explain using Le Chatelier's principle and in terms of forward and reverse reaction rates. [2] 2C(s) + 0,0) = 2 CO() Describe a situation when equilibrium concentrations change but no stress occurs. [1] Complete the following plots. The system is at equilibrium prior to t,. Atr, the volume of the system is suddenly doubled. The system responds to this stress between t, and t, until it re-equilibrates at ty. [2] N,0.0 2 NO,G) 1.2 INO) 10 0.8 0.6 INOJ 04 0.2 0.0 ts Time 9. Complete the following plots. The system below is at equilibrium prior to t. The system is suddenly cooled att The system responds to this stress between t, and t, until it re-equilibrates at ty [2] N,0,0) +57) = 2NO, 1.2 1.0 [NO) 08 0.6 9 IN,O) 04 02 OLD 13 Time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


