Question: plot can be shown in excel or matlab You are separating an ideal binary mixture of acetone and ethanol in a fractionating column. The mixture
plot can be shown in excel or matlab
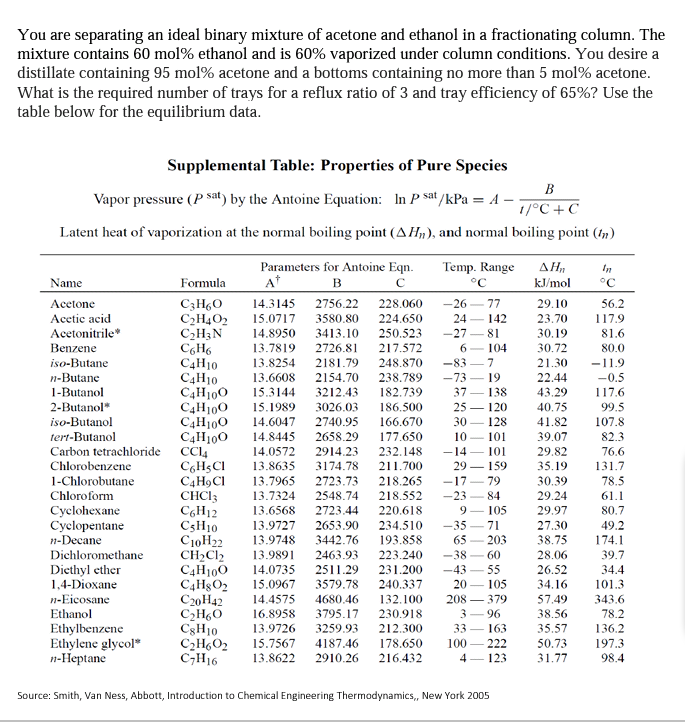
You are separating an ideal binary mixture of acetone and ethanol in a fractionating column. The mixture contains 60 mol% ethanol and is 60% vaporized under column conditions. You desire a distillate containing 95 mol% acetone and a bottoms containing no more than 5 mol% acetone. What is the required number of trays for a reflux ratio of 3 and tray efficiency of 65%? Use the table below for the equilibrium data. At C Supplemental Table: Properties of Pure Species B Vapor pressure (p sat) by the Antoine Equation: In p sat/kPa = A - 17C+C Latent heat of vaporization at the normal boiling point (AHn), and normal boiling point (In) Parameters for Antoine Eqn. Temp. Range , In Name Formula B C kJ/mol Acetone C3H60 14.3145 2756.22 228.060 -2677 29.10 56.2 Acetic acid C2H4O2 15.0717 3580.80 224.650 24 142 23.70 117.9 Acetonitrile C2H3N 14.8950 3413.10 250.523 -2781 30.19 81.6 Benzene CH 13.7819 2726.81 217.572 6-104 30.72 80.0 iso-Butane C4H10 13.8254 2181.79 248.870 -83-7 21.30 -11.9 n-Butane C4H10 13.6608 2154.70 238.789 -73 19 22.44 -0.5 1-Butanol C4H100 15.3144 3212.43 182.739 37 138 43.29 117.6 2-Butanol C4H100 15.1989 3026.03 186.500 25 - 120 40.75 99.5 iso-Butanol C4H100 14.6047 2740.95 166.670 30 128 41.82 107.8 tert-Butanol C4H100 14.8445 2658.29 177.650 10 101 39.07 82.3 Carbon tetrachloride CC14 14.0572 2914.23 232.148 -14 101 29.82 76.6 Chlorobenzene CHCI 13.8635 3174.78 211.700 29 159 35.19 131.7 1-Chlorobutane C4HACI 13.7965 2723.73 218.265 -1779 30.39 78.5 Chloroform CHCI: 13.7324 2548.74 218.552 -23 29.24 61.1 Cyclohexane CGH12 13.6568 2723.44 220.618 9 - 105 29.97 80.7 Cyclopentane CH10 13.9727 2653.90 234.510 -35-71 27.30 49.2 1-Decane C10H22 13.9748 3442.76 193.858 65 203 38.75 174.1 Dichloromethane CH2Cl2 13.9891 2463.93 223.240 -38 60 28.06 39.7 Diethyl ether C4H100 14.0735 2511.29 231.200 -43 55 26.52 34.4 1,4-Dioxane C4HgO2 15.0967 3579.78 240.337 20 105 34.16 101.3 --Eicosane C20H42 14.4575 4680.46 132.100 208 379 57.49 343.6 Ethanol C2H60 16.8958 3795.17 230.918 3 96 38.56 78.2 Ethylbenzene CgH10 13.9726 3259.93 212.300 33 163 35.57 136.2 Ethylene glycol* C2H602 15.7567 4187.46 178.650 100 50.73 197.3 1-Heptane C7H16 13.8622 2910.26 216.432 4 123 31.77 98.4 84 Source: Smith, Van Ness, Abbott, Introduction to Chemical Engineering Thermodynamics, New York 2005
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


