Question: Plot the flame temperature and total entropy (or entropy difference) for burning H2 and gaseous oxygen as a function of the dissociation of H2O into
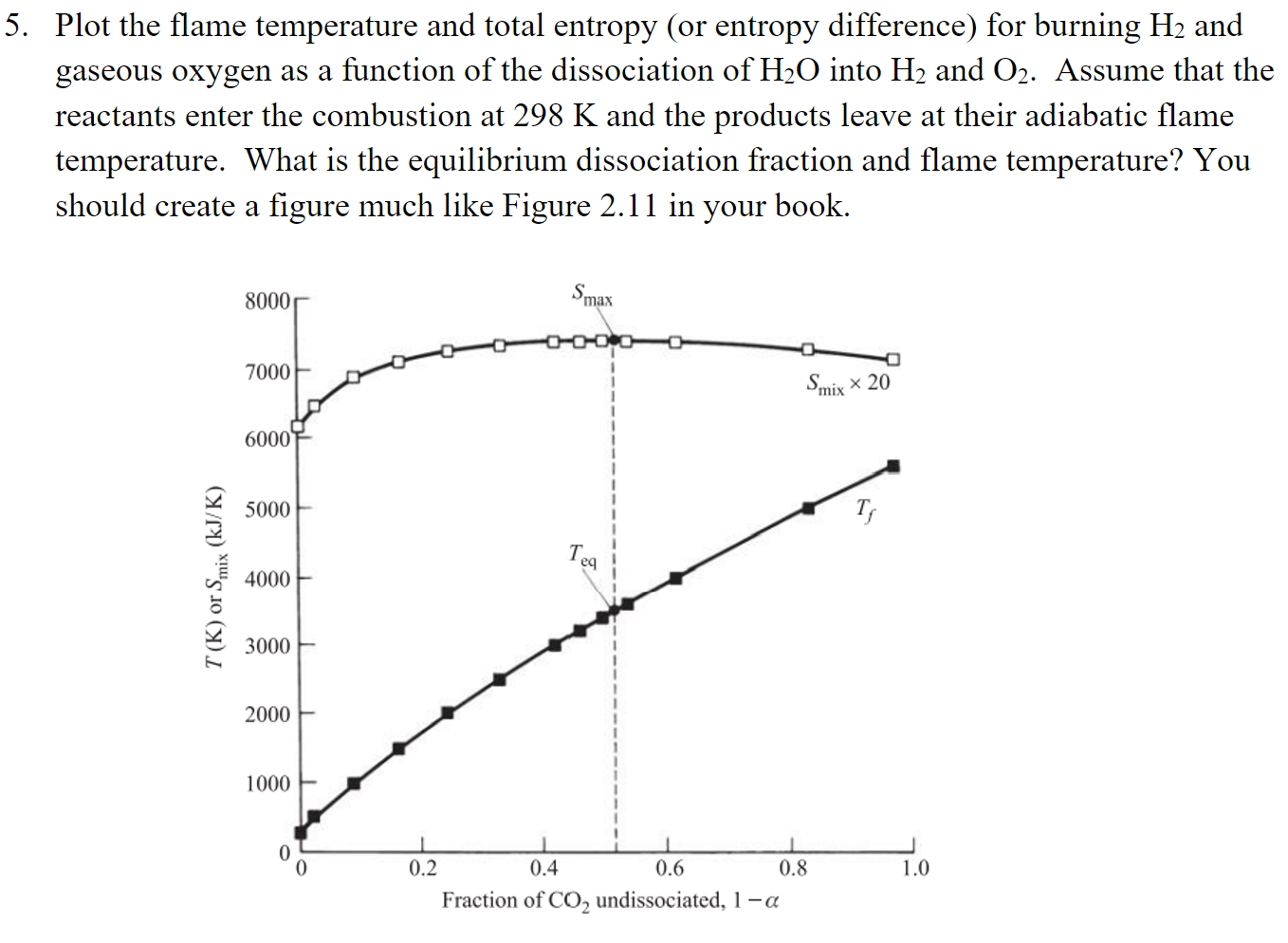
Plot the flame temperature and total entropy (or entropy difference) for burning H2 and gaseous oxygen as a function of the dissociation of H2O into H2 and O2. Assume that the reactants enter the combustion at 298K and the products leave at their adiabatic flame temperature. What is the equilibrium dissociation fraction and flame temperature? You should create a figure much like Figure 2.11 in your book
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


