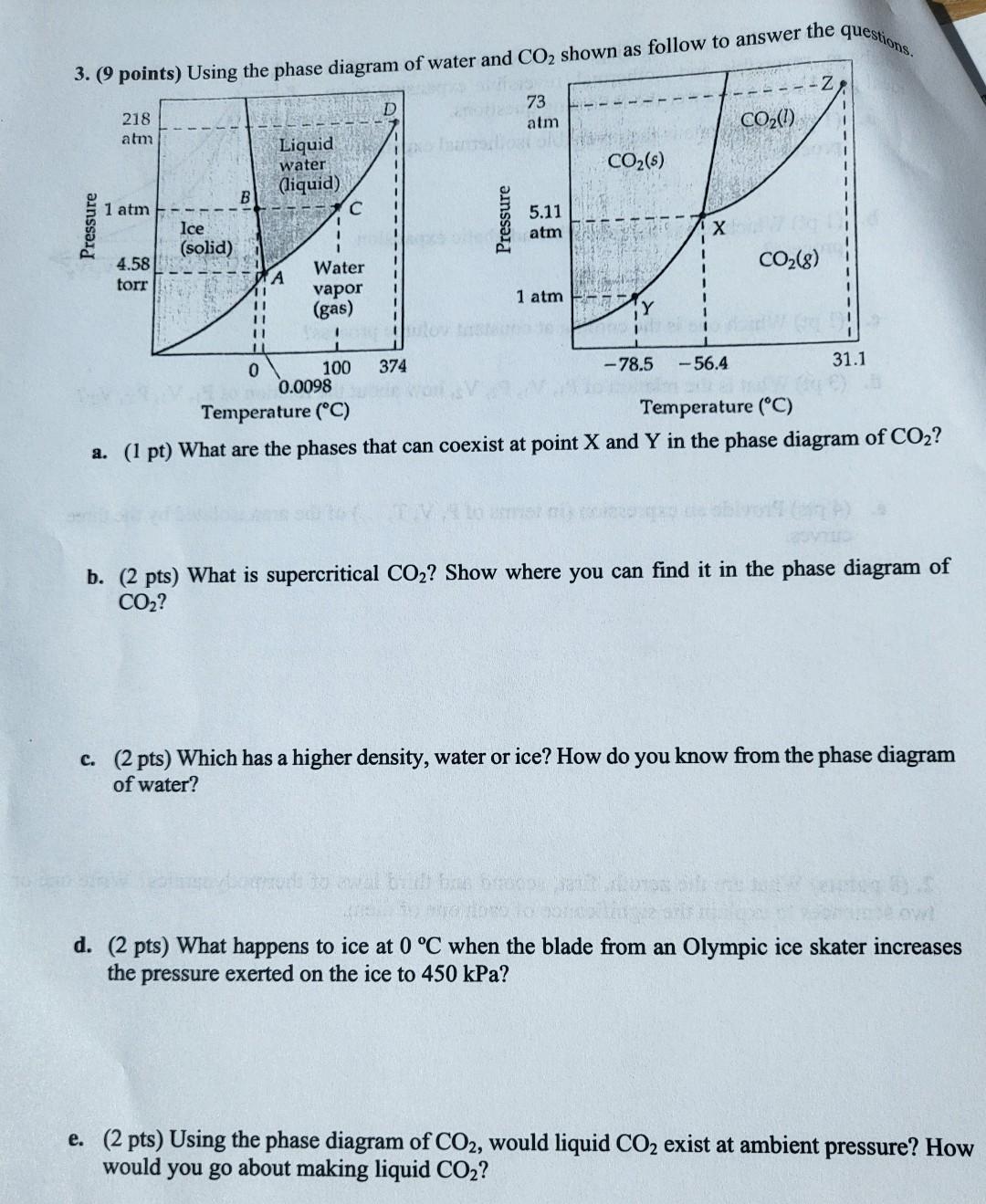
Question: pls answer all parts questions. 3. (9 points) Using the phase diagram of water and CO2 shown as follow to answer the -Z D 218
pls answer all parts
questions. 3. (9 points) Using the phase diagram of water and CO2 shown as follow to answer the -Z D 218 atm 73 atm CO2(!) Liquid (liquid) water CO2(s) 1 1 atm Pressure Pressure 5.11 atm Ice (solid) CO2(8) 4.58 torr A Water vapor (gas) 11 11 11 11 1 atm 0 374 31.1 100 -78.5 -56.4 0.0098 Temperature (C) Temperature (C) a. (1 pt) What are the phases that can coexist at point X and Y in the phase diagram of CO2? b. (2 pts) What is supercritical CO2? Show where you can find it in the phase diagram of CO2? c. (2 pts) Which has a higher density, water or ice? How do you know from the phase diagram of water? Poloko d. (2 pts) What happens to ice at 0 C when the blade from an Olympic ice skater increases the pressure exerted on the ice to 450 kPa? e. (2 pts) Using the phase diagram of CO2, would liquid CO2 exist at ambient pressure? How would you go about making liquid CO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


