Question: pls answer fast Question 23 (1 point) The steam reforming reaction of methane follows this chemical equation: CH4(g)+H2O(g)CO(g)+3H2(g)rH=+194kJ/mol At 298K the reaction lies far to
pls answer fast 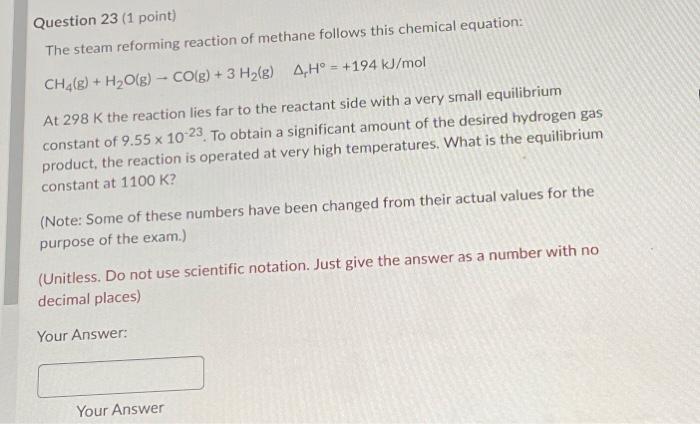

Question 23 (1 point) The steam reforming reaction of methane follows this chemical equation: CH4(g)+H2O(g)CO(g)+3H2(g)rH=+194kJ/mol At 298K the reaction lies far to the reactant side with a very small equilibrium constant of 9.551023. To obtain a significant amount of the desired hydrogen gas product, the reaction is operated at very high temperatures. What is the equilibrium constant at 1100K ? (Note: Some of these numbers have been changed from their actual values for the purpose of the exam.) (Unitless. Do not use scientific notation. Just give the answer as a number with no decimal places) Your
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


