Question: pls answer. will upvote CHE CALCULATIONS 2 1. Given the following composition of the fuel gas 9.2%CO2,0.4%CH2H4,20.9%CO,15.6%H2,1.9% CH4, and 52%N2. The flue gas has the
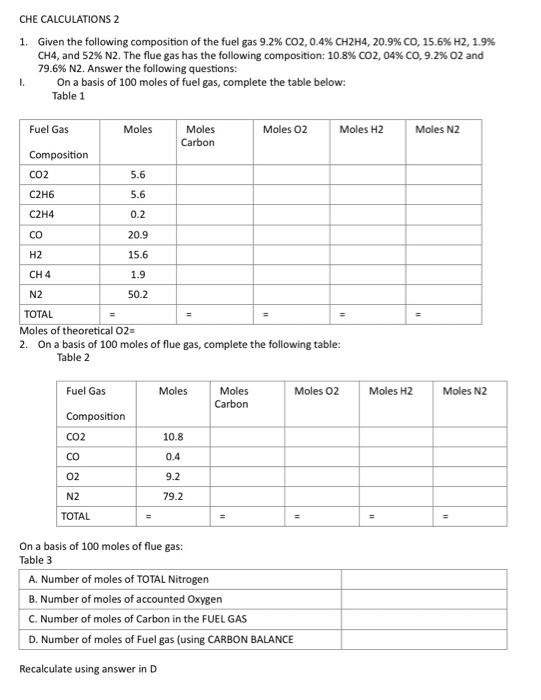
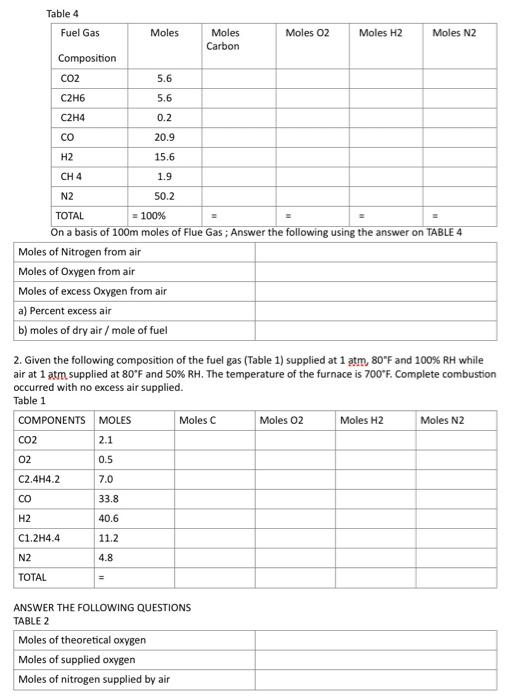
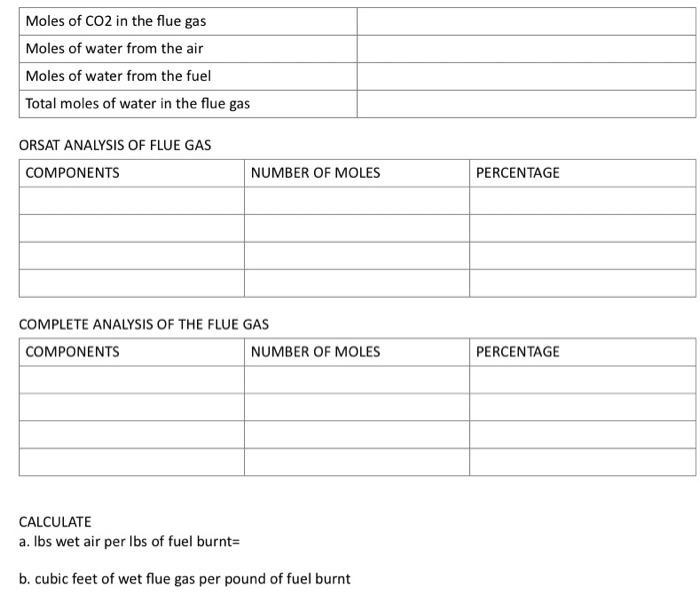
CHE CALCULATIONS 2 1. Given the following composition of the fuel gas 9.2%CO2,0.4%CH2H4,20.9%CO,15.6%H2,1.9% CH4, and 52%N2. The flue gas has the following composition: 10.8%CO2,04%CO,9.2%O2 and 79.6\% N2. Answer the following questions: I. On a basis of 100 moles of fuel gas, complete the table below: Table 1 2. On a basis of 100 moles of flue gas, complete the following table: Table 2 On a basis of 100 moles of flue gas: Tahlo 2 Recalculate using answer in D 2. Given the following composition of the fuel gas (Table 1) supplied at 1atm,80F and 100%RH while air at 1atm supplied at 80F and 50%RH. The temperature of the furnace is 700F. Complete combustion occurred with no excess air supplied. Table 1 ANSWER THE FOLLOWING QUESTIONS TABLE 2 ORSAT ANALYSIS OF FLUE GAS COMPLETE ANALYSIS OF THE FLUE GAS CALCULATE a. lbs wet air per Ibs of fuel burnt= b. cubic feet of wet flue gas per pound of fuel burnt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


