Question: pls do not combine terms Consider the equilibrium system described by the chemical reaction below. At equilibrium, a sample of gas from the system is
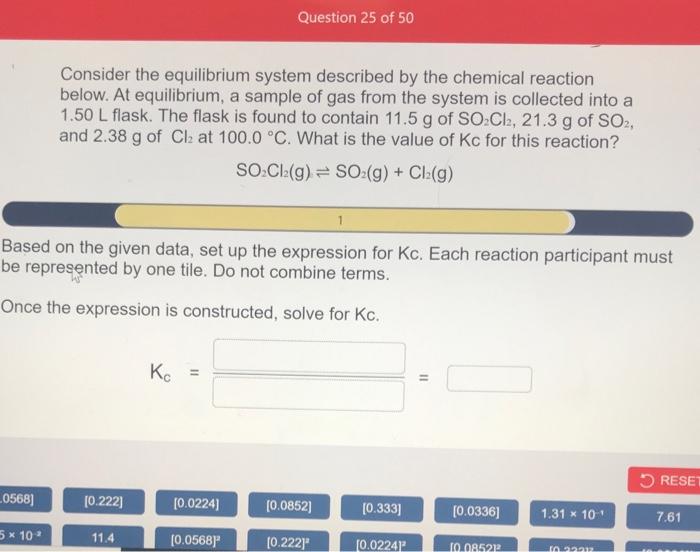
Consider the equilibrium system described by the chemical reaction below. At equilibrium, a sample of gas from the system is collected into a 1.50L flask. The flask is found to contain 11.5g of SO2Cl2,21.3gofSO2, and 2.38g of Cl2 at 100.0C. What is the value of Kc for this reaction? SO2Cl2(g)SO2(g)+Cl2(g) 3ased on the given data, set up the expression for Kc. Each reaction participant must e represented by one tile. Do not combine terms. Once the expression is constructed, solve for Kc. KC=i=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


