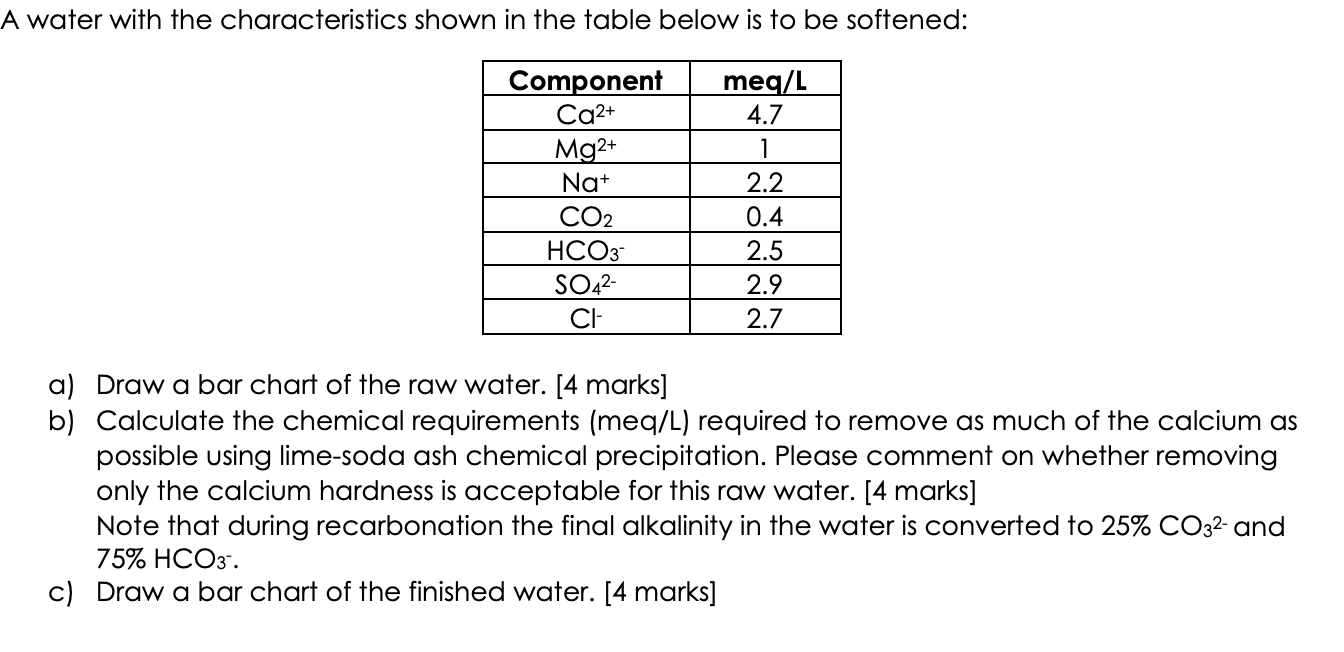
Question: pls explain in depth how you found CO 2 , soda ash and lime doses. And also how to treat that maximum calcium removal.A water
pls explain in depth how you found CO soda ash and lime doses. And also how to treat that maximum calcium removal.A water with the characteristics shown in the table below is to be softened:
a Draw a bar chart of the raw water. marks
b Calculate the chemical requirements meqL required to remove as much of the calcium as
possible using limesoda ash chemical precipitation. Please comment on whether removing
only the calcium hardness is acceptable for this raw water. marks
Note that during recarbonation the final alkalinity in the water is converted to and
c Draw a bar chart of the finished water. marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


