Question: pls explain with steps Calculate the change in entropy when 193. of water at 70.2 C is mixed into 193. g of water at 21.4
pls explain with steps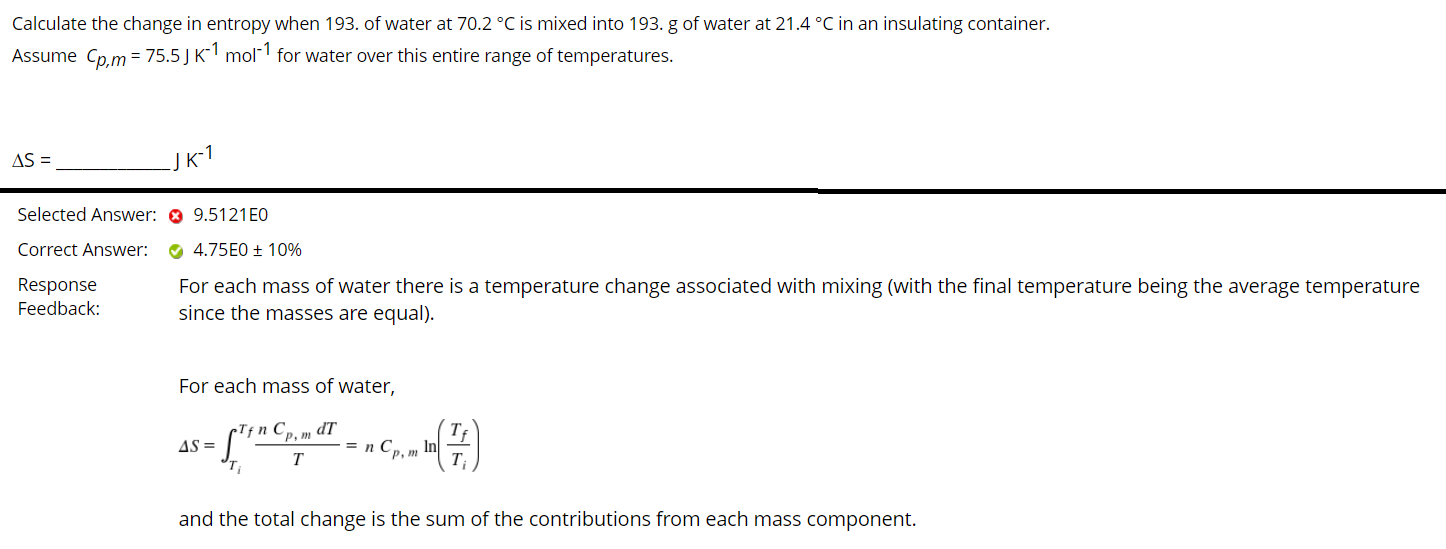
Calculate the change in entropy when 193. of water at 70.2 C is mixed into 193. g of water at 21.4 C in an insulating container. Assume Cp,m= 75.5) K-1 mol-1 for water over this entire range of temperatures. AS = JK-1 Selected Answer: 9.5121E0 Correct Answer: Response Feedback: 4.75E0 + 10% For each mass of water there is a temperature change associated with mixing (with the final temperature being the average temperature since the masses are equal). For each mass of water, np dT AS = - ""Cance (7) Sincy , Inl T and the total change is the sum of the contributions from each mass component
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


