Question: PLS HELP 1 - 5 POSTLAB QUESTIONS 1 . From your results in Part A , can a prediction be made about the nature of
PLS HELP POSTLAB QUESTIONS
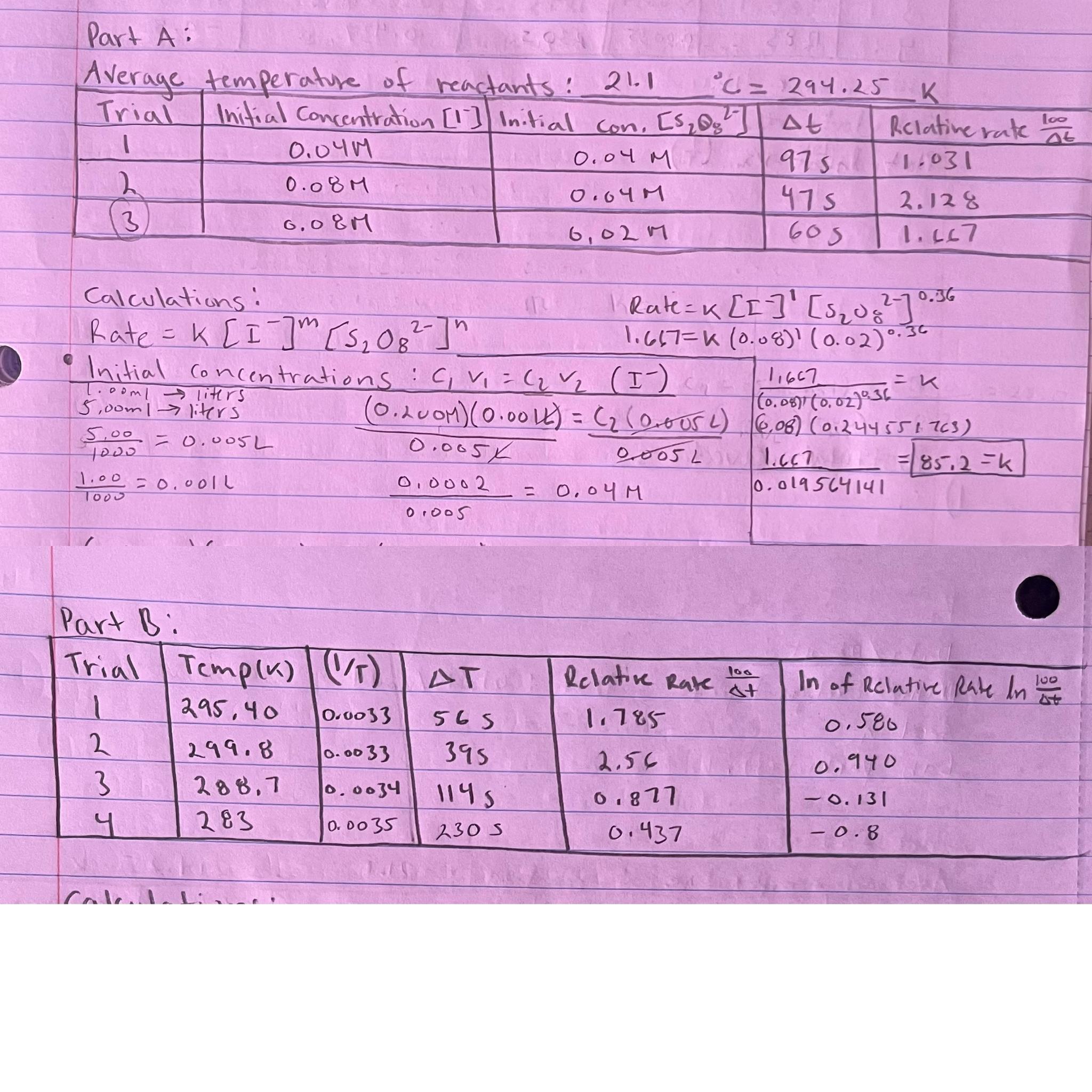
From your results in Part A can a prediction be made about the nature of the ratecontrolling step in this reaction? If not, why not? If so give a plausible chemical equation of this step Hint: Think about the connection between the molecularity of the ratecontrolling step and the order of the overall reaction
From your results in Part A calculate the time for the blue color to appear for this system if mL KI mL KCl mL NHSO and mL NaSO from Table are mixed. Using your linear plot In Delta T vsT in Part B measure the experimental slope and use it to calculate the activation energy, EA in units of kJmol
Use your data from Part B and the Arrhenius equation to calculate the value of EA for this reaction. How does this value compare with the obtained value in number the slope from the graph Which do you think is more accurate and why? Note: we used relative rate for k In kkEaR TTTT Does your data agree with the postulate that the reaction rate will double for a oC rise in temperature? Support your answer with a calculation.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


