Question: pls help i keep getting it wrong :( Consider a general reaction A(aq)encymeB(aq) The G of the reaction is 9.240kJmol1. Calculate the equilibrium constant for
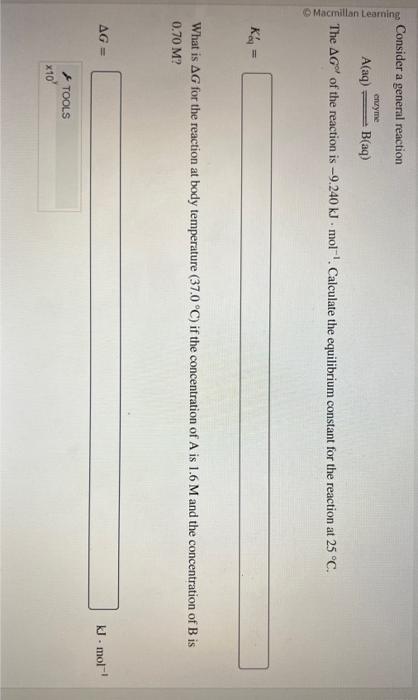
Consider a general reaction A(aq)encymeB(aq) The G of the reaction is 9.240kJmol1. Calculate the equilibrium constant for the reaction at 25C. Keq= What is G for the reaction at body temperature (37.0C) if the concentration of A is 1.6M and the concentration of B is 0.70M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


